Page 92 - Soil and water contamination, 2nd edition
P. 92
Solid phase constituents 79
a . b c
6642 6642 6642
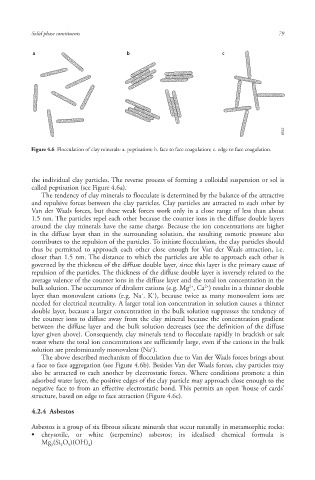
Figure 4.6 Flocculation of clay minerals: a. peptisation; b. face to face coagulation; c. edge to face coagulation.
the individual clay particles. The reverse process of forming a colloidal suspension or sol is
called peptisation (see Figure 4.6a).
The tendency of clay minerals to flocculate is determined by the balance of the attractive
and repulsive forces between the clay particles. Clay particles are attracted to each other by
Van der Waals forces , but these weak forces work only in a close range of less than about
1.5 nm. The particles repel each other because the counter ions in the diffuse double layers
around the clay minerals have the same charge. Because the ion concentrations are higher
in the diffuse layer than in the surrounding solution, the resulting osmotic pressure also
contributes to the repulsion of the particles. To initiate flocculation , the clay particles should
thus be permitted to approach each other close enough for Van der Waals attraction, i.e.
closer than 1.5 nm. The distance to which the particles are able to approach each other is
governed by the thickness of the diffuse double layer, since this layer is the primary cause of
repulsion of the particles. The thickness of the diffuse double layer is inversely related to the
average valence of the counter ions in the diffuse layer and the total ion concentration in the
2+
2+
bulk solution. The occurrence of divalent cations (e.g. Mg , Ca ) results in a thinner double
+
+
layer than monovalent cations (e.g. Na , K ), because twice as many monovalent ions are
needed for electrical neutrality. A larger total ion concentration in solution causes a thinner
double layer, because a larger concentration in the bulk solution suppresses the tendency of
the counter ions to diffuse away from the clay mineral because the concentration gradient
between the diffuse layer and the bulk solution decreases (see the definition of the diffuse
layer given above). Consequently, clay minerals tend to flocculate rapidly in brackish or salt
water where the total ion concentrations are sufficiently large, even if the cations in the bulk
+
solution are predominantly monovalent (Na ).
The above described mechanism of flocculation due to Van der Waals forces brings about
a face to face aggregation (see Figure 4.6b). Besides Van der Waals forces, clay particles may
also be attracted to each another by electrostatic forces. Where conditions promote a thin
adsorbed water layer, the positive edges of the clay particle may approach close enough to the
negative face to from an effective electrostatic bond. This permits an open ‘house of cards’
structure, based on edge to face attraction (Figure 4.6c).
4.2.4 Asbestos
Asbestos is a group of six fibrous silicate minerals that occur naturally in metamorphic rocks:
• chrysotile, or white ( serpentine) asbestos; its idealised chemical formula is
Mg (Si O )(OH) )
3 2 5 4
10/1/2013 6:44:23 PM
Soil and Water.indd 91 10/1/2013 6:44:23 PM
Soil and Water.indd 91