Page 97 - Soil and water contamination, 2nd edition
P. 97
84 Soil and Water Contamination
a COO - COO OH 2 6642 6642 6642
+ Cu + Cu
OH O OH 2
2- 2+
b c
NH 2 OH 2
COO OOC
H C
Cu 2
Cu
O O
H C
2
NH OH 2
2
COO OH -
d e
Fe COO OH
O (OH ) n-1 Fe (H O) n-2
2
2
O OH
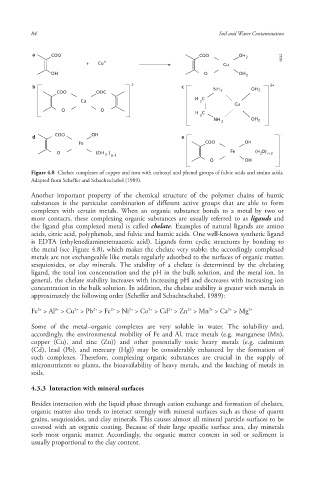
Figure 4.8 Chelate complexes of copper and iron with carboxyl and phenol groups of fulvic acids and amino acids.
Adapted from Scheffer and Schachtschabel (1989).
Another important property of the chemical structure of the polymer chains of humic
substances is the particular combination of different active groups that are able to form
complexes with certain metals . When an organic substance bonds to a metal by two or
more contacts, these complexing organic substances are usually referred to as ligands and
the ligand plus complexed metal is called chelate . Examples of natural ligands are amino
acids, citric acid, polyphenols, and fulvic and humic acids . One well-known synthetic ligand
is EDTA (ethylenediaminetetraacetic acid). Ligands form cyclic structures by bonding to
the metal (see Figure 4.8), which makes the chelate very stable; the accordingly complexed
metals are not exchangeable like metals regularly adsorbed to the surfaces of organic matter ,
sesquioxides , or clay minerals . The stability of a chelate is determined by the chelating
ligand, the total ion concentration and the pH in the bulk solution, and the metal ion. In
general, the chelate stability increases with increasing pH and decreases with increasing ion
concentration in the bulk solution. In addition, the chelate stability is greater with metals in
approximately the following order (Scheffer and Schachtschabel, 1989):
2+
2+
2+
2+
2+
2+
2+
2+
3+
3+
2+
Fe > Al > Cu > Pb > Fe > Ni > Co > Cd > Zn > Mn > Ca > Mg 2+
Some of the metal–organic complexes are very soluble in water. The solubility and,
accordingly, the environmental mobility of Fe and Al, trace metals (e.g. manganese (Mn),
copper (Cu), and zinc (Zn)) and other potentially toxic heavy metals (e.g. cadmium
(Cd), lead (Pb), and mercury (Hg)) may be considerably enhanced by the formation of
such complexes. Therefore, complexing organic substances are crucial in the supply of
micronutrients to plants, the bioavailability of heavy metals , and the leaching of metals in
soils.
4.3.3 Interaction with mineral surfaces
Besides interaction with the liquid phase through cation exchange and formation of chelates,
organic matter also tends to interact strongly with mineral surfaces such as those of quartz
grains, sesquioxides , and clay minerals . This causes almost all mineral particle surfaces to be
covered with an organic coating. Because of their large specific surface area, clay minerals
sorb most organic matter. Accordingly, the organic matter content in soil or sediment is
usually proportional to the clay content .
10/1/2013 6:44:23 PM
Soil and Water.indd 96 10/1/2013 6:44:23 PM
Soil and Water.indd 96