Page 101 - Soil and water contamination, 2nd edition
P. 101
88 Soil and Water Contamination
amount of oxygen consumed in the complete oxidation of carbonaceous matter, thus also the
non-biodegradable matter. In rivers and lakes , a significant part of the oxygen consumption
is due to the decomposition of organic matter deposited in the bed sediments . The sediment
oxygen demand (SOD) is the rate of the dissolved oxygen consumption in a water body
(river, lake or ocean) due to the decomposition of sediment organic matter. The SOD
-1
-2
is expressed in g O m d . The BOD, COD, and SOD are often important parameters
2
of the dissolved oxygen budget of surface waters and their determination provides crucial
information for adequate water quality control.
4.4 SORPTION BY SOILS AND SEDIMENTS
In the previous sections, it was observed that both the inorganic and the organic components
in soils and sediments have or can have negatively charged surface sites and so are able to
adsorb cations . The CEC of clay minerals varies from less than 10 meq/100 g (kaolinite ) to
more than 100 meq/100 g (montmorillonite and vermiculite ) (see Table 4.2) and the CEC of
organic matter ranges between 100 and 300 meq/100 g. The CEC of sesquioxides typically has
a much smaller value and is commonly less than 3 meq/100 g soil. All these constituents thus
contribute to the CEC of the bulk soil. Obviously, the CEC of a soil is closely related to its
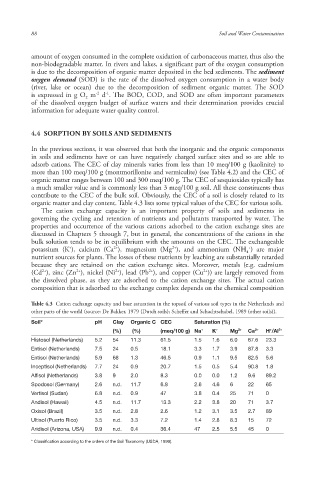
organic matter and clay content . Table 4.3 lists some typical values of the CEC for various soils.
The cation exchange capacity is an important property of soils and sediments in
governing the cycling and retention of nutrients and pollutants transported by water. The
properties and occurrence of the various cations adsorbed to the cation exchange sites are
discussed in Chapters 5 through 7, but in general, the concentrations of the cations in the
bulk solution tends to be in equilibrium with the amounts on the CEC . The exchangeable
2+
2+
+
+
potassium (K ), calcium (Ca ), magnesium (Mg ), and ammonium (NH ) are major
4
nutrient sources for plants. The losses of these nutrients by leaching are substantially retarded
because they are retained on the cation exchange sites. Moreover, metals (e.g. cadmium
2+
2+
2+
2+
2+
(Cd ), zinc (Zn ), nickel (Ni ), lead (Pb ), and copper (Cu )) are largely removed from
the dissolved phase , as they are adsorbed to the cation exchange sites. The actual cation
composition that is adsorbed to the exchange complex depends on the chemical composition
Table 4.3 Cation exchange capacity and base saturation in the topsoil of various soil types in the Netherlands and
other parts of the world (source: De Bakker, 1979 (Dutch soils); Scheffer and Schachtschabel, 1989 (other soils)).
Soil* pH Clay Organic C CEC Saturation (%)
+
(%) (%) (meq/100 g) Na + K + Mg 2+ Ca 2+ H /Al 3+
Histosol (Netherlands) 5.2 54 11.3 61.5 1.5 1.6 6.0 67.6 23.3
Entisol (Netherlands) 7.5 24 0.5 18.1 3.3 1.7 3.9 87.8 3.3
Entisol (Netherlands) 5.9 68 1.3 46.5 0.9 1.1 9.5 82.5 5.6
Inceptisol (Netherlands) 7.7 24 0.9 20.7 1.5 0.5 5.4 90.8 1.8
Alfisol (Netherlands) 3.8 9 2.0 8.3 0.0 0.0 1.2 9.6 89.2
Spodosol (Germany) 2.6 n.d. 11.7 6.8 2.6 4.6 6 22 65
Vertisol (Sudan) 6.8 n.d. 0.9 47 3.8 0.4 25 71 0
Andisol (Hawaii) 4.5 n.d. 11.7 13.3 2.2 3.8 20 71 3.7
Oxisol (Brazil) 3.5 n.d. 2.8 2.6 1.2 3.1 3.5 2.7 89
Ultisol (Puerto Rico) 3.5 n.d. 3.3 7.2 1.4 2.8 8.3 15 72
Aridisol (Arizona, USA) 9.9 n.d. 0.4 36.4 47 2.5 5.5 45 0
* Classification according to the orders of the Soil Taxonomy (USDA, 1999).
10/1/2013 6:44:24 PM
Soil and Water.indd 100
Soil and Water.indd 100 10/1/2013 6:44:24 PM