Page 105 - Soil and water contamination, 2nd edition
P. 105
92 Soil and Water Contamination
Table 5.1 Typical concentration ranges of major dissolved constituents in unpolluted fresh water and their sources
(source: Appelo and Postma, 1996).
Dissolved Typical concentration range Source
constituent mmol l mg l -1
-1
Na + 0.1–2 0.2–46 Feldspar , rock salt, zeolite, atmosphere
K + 0.01– 0.2 0.4–8 Feldspar, micas
Ca 2+ 0.05–5 2–200 Limestone, gypsum feldspar, pyroxene, amphibole
Mg 2+ 0.05–2 1.2–48 Dolomite, serpentine, pyroxene, amphibole, olivine, micas
Cl - 0.05–2 1.8–70 Rock salt, atmosphere
- 0–5 0–305 Limestone, organic matter
HCO 3
- 0.01–5 1–480 Gypsum , sulphides, atmosphere
SO 4
Fe 2+ 0–0.5 0–28 Silicates, goethite, haematite , siderite, pyrite
0.02–1 1.2– 60 Silicates
SiO 2
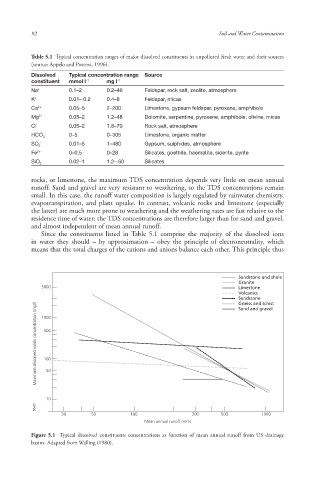
rocks, or limestone , the maximum TDS concentration depends very little on mean annual
runoff. Sand and gravel are very resistant to weathering, so the TDS concentrations remain
small. In this case, the runoff water composition is largely regulated by rainwater chemistry,
evapotranspiration , and plant uptake . In contrast, volcanic rocks and limestone (especially
the latter) are much more prone to weathering and the weathering rates are fast relative to the
residence time of water; the TDS concentrations are therefore larger than for sand and gravel,
and almost independent of mean annual runoff.
Since the constituents listed in Table 5.1 comprise the majority of the dissolved ions
in water they should – by approximation – obey the principle of electroneutrality , which
means that the total charges of the cations and anions balance each other. This principle thus
Sandstone and shale
Granite
5000 Limestone
Volcanics
Sandstone
Maximum dissolved solids concentration (mg/l) 500
Gneiss and schist
Sand and gravel
1000
100
50
10
6642 6642 6642
30 50 100 300 500 1000
Mean annual runoff (mm)
Figure 5.1 Typical dissolved constituents concentrations as function of mean annual runoff from US drainage
basins. Adapted from Walling (1980).
10/1/2013 6:44:24 PM
Soil and Water.indd 104
Soil and Water.indd 104 10/1/2013 6:44:24 PM