Page 108 - Soil and water contamination, 2nd edition
P. 108
Major dissolved phase constituents 95
1 6
Na+K Cl Na+K Cl
Ca HCO3 Ca HCO3
Mg SO4 Mg SO4
Fe NO3 Fe NO3
25 20 15 10 5 0 5 10 15 20 25 meq/l 50 40 30 20 10 0 10 20 30 40 50 meq/l
25
2 7
Na+K Cl Na+K Cl
Ca HCO3 Ca HCO3
Mg SO4 Mg SO4
Fe NO3 Fe NO3
1.0 0.8 0.6 0.4 0.2 0.0 0.2 0.4 0.6 0.8 1.0 meq/l 10 8 6 4 2 0 2 4 6 8 10 meq/l
3 8
Na+K Cl Na+K Cl
Ca HCO3 Ca HCO3
Mg SO4 Mg SO4
Fe NO3 Fe NO3
100 80 60 40 20 0 20 40 60 80 100 meq/l 250 200 150 100 50 0 50 100 150 200 250meq/l
4 9
Na+K Cl Cl Na+K Cl
Na+K
HCO3
Ca HC O 3 Ca HCO3
Mg SO4 4 Mg SO4
SO
Fe NO3 Fe NO3
NO3
meq/l
25 20 15 10 5 0 5 10 15 20 25 meq/l 2.5 2.0 1.5 1.0 0.5 0.0 0.5 1.0 1.5 2.0 2.5 meq/l
5 10
Na+K Cl Na+K Cl
Ca HCO3 Ca HCO3
Mg SO4 Mg SO4
Fe NO3 Fe NO3
6642 6642 6642 5 4 3 2 1 0 1 2 3 4 5 meq/l 1.0 0.8 0.6 0.4 0.2 0.0 0.2 0.4 0.6 0.8 1.0 meq/l
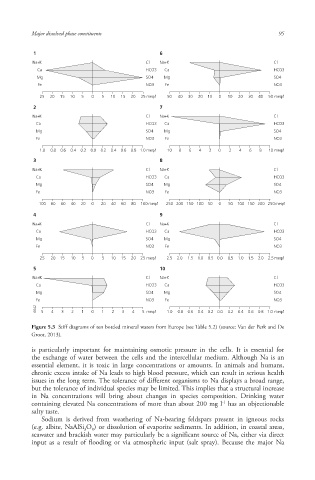
Figure 5.3 Stiff diagrams of ten bottled mineral waters from Europe (see Table 5.2) (source: Van der Perk and De
Groot, 2013).
is particularly important for maintaining osmotic pressure in the cells. It is essential for
the exchange of water between the cells and the intercellular medium. Although Na is an
essential element, it is toxic in large concentrations or amounts. In animals and humans,
chronic excess intake of Na leads to high blood pressure, which can result in serious health
issues in the long term. The tolerance of different organisms to Na displays a broad range,
but the tolerance of individual species may be limited. This implies that a structural increase
in Na concentrations will bring about changes in species composition. Drinking water
-1
containing elevated Na concentrations of more than about 200 mg l has an objectionable
salty taste.
Sodium is derived from weathering of Na-bearing feldspars present in igneous rocks
(e.g. albite, NaAlSi O ) or dissolution of evaporite sediments. In addition, in coastal areas,
3 8
seawater and brackish water may particularly be a significant source of Na, either via direct
input as a result of flooding or via atmospheric input (salt spray). Because the major Na
10/1/2013 6:44:24 PM
Soil and Water.indd 107
Soil and Water.indd 107 10/1/2013 6:44:24 PM