Page 400 - Standard Handbook Petroleum Natural Gas Engineering VOLUME2
P. 400
366 Production
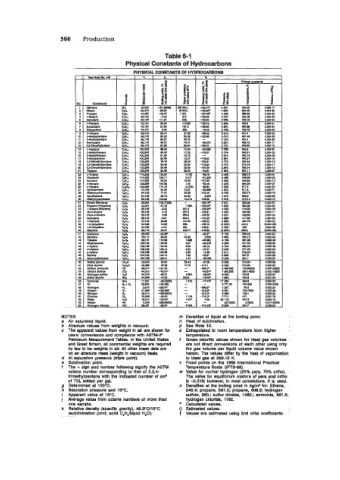
Table 6-1
Physical Constants of Hydrocarbons
YS
r -
1.
I
a
1
NO.
1 16m3
2 aamo
3 um7
4
58d91
6 - -
58.i7A
6 72161
8 - -
72181
7
72in
0 ea178
10 88176
11 8L17a
13 - -
12
88.178
a178
14 IMLZOS
15 imm
18 imm
17 1-
18 1-
19 imm
21 - -
20
imp06
imam
22 ll42Sz
28 11-
7A 114m
25 1-
aB 1-
n M.lSS
28 e41 a
es
- -
81.162
98.198
80
31 28.m
32 42081
33 ffl1M
81 mica
86 mlB
88 aaio8
37 70.136
88 54.092
so -
YDep
40 -
6311s
41 !ac@a
42 78114
4s 62141
44 imiea
45 lrnlea
45 1rn188
47 lOLldB
48
48 - -
104692
120.1%
32Ca
51 4a.m
92 mmo
IS umo
Y
- 34.076
I
&%OM
I 17.081
57 zBK4
68 2018
59 31.998
211.M3
80 -
81 7asw
M la015
*ow
I -
ed
NOTES m Densities of liquid at the boillng point.
a Air saturated Ilquid. n Heat of sublimation.
b Absolute values from welghts in vacuum. p See Note 10.
c The apparent values from welght in air are shown for 8 Extrapolated to room temperature from hlgher
users' convenience and mmpllanm with ASTM-IP temperature.
Petmleum Measurement Tables. In the United States t Gross calorific values shown for ideal gas volumes
and Great Britain, all cornmemiel welghts are requlred are not direct converslons of each other uslng only
by law to be weights In air. All other maas data are the gas volume per Iiquld volume value shown
on an absolute mass (welght in vacuum) basls. herein. The values differ by the heat of vaporization
d At saturatlon pressure (trlple polnt). to ideal gas at 288.15 K.
e Sublimation point. Y Fixed polnts on the 1968 International Practical
f The + sign and number following signify the ASTM Temperature Scale (IPTS-88).
octane number corresponding to that of 2.2.4- w Value for normal hydrogen (25% para, 75% ortho).
trimethylpentane wlth the Indicated number of cd The value for equillbrlum mixture of para and ortho
of TEL added per gal. is -0.218; however, in most correlations, 0 is used.
g Determlned at 100°C. x Densities at the boiling point In kg/nP for: Ethane,
h Saturation pressure and 15°C. 548.4; propane, 581.0; propene, 608.8; hydrogen
I Apparent value at 15%. sulflde, 960.: sulfur dloxide, 1482.; ammonia. 881.6
j Average value from octane numbere of more than hydrogen chloride, 1192.
one sample. Calculated values.
k Relatlve density (speclfii gravity), 48.8°C/150C () Estimated values.
(subllminatlon polnt; solld C,H,Alquid H,O). t Values are estimated using 2nd vlrlal coefflclents.