Page 238 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 238
CASE STUDIES OF ELECTROSTATIC PROPERTIES OF SMALL MOLECULES 221
potential have been chosen as C(5,3;5,3), O(4,4;4,4), N(4,3;4,3) and H(3,l;3,l).
The ab initio calculations have been performed using the Gaussian 90 program package
[18]. The 6-31+G(2d,2p) has been employed throughout at both self-consistent field (SCF)
and MP2 levels of theory. In all the calculations, the proton affinities (PAs) have been
obtained as the difference between total energies of optimized unprotonated and protonated
species and no zero-point nor thermodynamic contributions have been introduced. This
means that we are more interested in comparing the PAs deduced from the theoretical
models at various levels of theory than in performing accurate comparisons with the
experimental values.
3. Results and Discussion
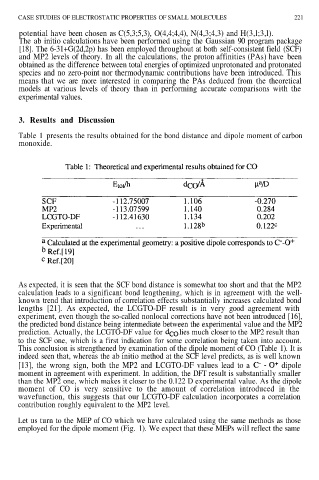
Table 1 presents the results obtained for the bond distance and dipole moment of carbon
monoxide.
As expected, it is seen that the SCF bond distance is somewhat too short and that the MP2
calculation leads to a significant bond lengthening, which is in agreement with the well-
known trend that introduction of correlation effects substantially increases calculated bond
lengths [21]. As expected, the LCGTO-DF result is in very good agreement with
experiment, even though the so-called nonlocal corrections have not been introduced [16],
the predicted bond distance being intermediate between the experimental value and the MP2
prediction. Actually, the LCGTO-DF value for lies much closer to the MP2 result than
to the SCF one, which is a first indication for some correlation being taken into account.
This conclusion is strengthened by examination of the dipole moment of CO (Table 1). It is
indeed seen that, whereas the ab initio method at the SCF level predicts, as is well known
[13], the wrong sign, both the MP2 and LCGTO-DF values lead to a dipole
moment in agreement with experiment. In addition, the DFT result is substantially smaller
than the MP2 one, which makes it closer to the 0.122 D experimental value. As the dipole
moment of CO is very sensitive to the amount of correlation introduced in the
wavefunction, this suggests that our LCGTO-DF calculation incorporates a correlation
contribution roughly equivalent to the MP2 level.
Let us turn to the MEP of CO which we have calculated using the same methods as those
employed for the dipole moment (Fig. 1). We expect that these MEPs will reflect the same