Page 239 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 239
222 J. WEBER ET AL.
trend as the dipole moment, inasmuch as a multicenter multipole expansion of the molecular
electron charge density can be used to generate a MEP which is a good approximation to
that obtained from the wavefunction [22]. It has been shown indeed that the contributions
arising from the monopolar and dipolar terms are generally preponderant [23], which
suggests that the MEP and molecular dipole moment calculated from the same wavefunction
should exhibit the same trend within a series of compounds or when evaluated at various
levels of theory.
It is seen in Fig. la that the MEP calculated at the ab initio SCF level exhibits two roughly
equivalent minima on both carbon and oxygen ends, lying at -14.0 and -11.4 kcal/mol,
respectively, along the CO bond axis. From the point of view of electrostatics, both atoms
behave therefore similarly at the SCF level towards an incoming proton. This picture is
drastically modified when examining the MP2 result (Fig. 1b). In this case, the minimum
on carbon (-18.0 kcal/mol) is significantly lower than that on oxygen (-3.8 kcal/mol),
which indicates that the electron density calculated at the MP2 level is substantially different
from that resulting from the SCF calculation. In particular, this dissymetry in the MEP
minima on both atoms suggests that part of the electron density is shifted towards the
carbon atom, which simultaneously allows to rationalize the change of sign of the calculated
dipole moment when going from SCF to MP2 and the polarity evaluated in this
latter case. It is therefore of interest to examine the LCGTO-DF MEP (Fig. 1c) in order to
confirm the shift of electron density induced by introduction of correlation. It is seen that
indeed the LCGTO-DF result is very similar to the MP2 map, with MEP minima lying at
-18.8 kcal/mol on the C-end at -6.7 kcal/mol on the O-end. However, the difference
between C and O MEP minima is about 2 kcal/mol larger in MP2 than in LCGTO-DF,
which suggests that the MP2 electron density of CO leads to a slightly more polar molecule
than in the LCGTO-DF case and hence to a larger dipole moment (see Table 1). In
spite of these small differences, there is no doubt when examining Fig. 1 that the LCGTO-
DF electron density calculated for CO incorporates correlation to an extent comparable to
MP2, which confirms the results of previous investigations [4,9,16].
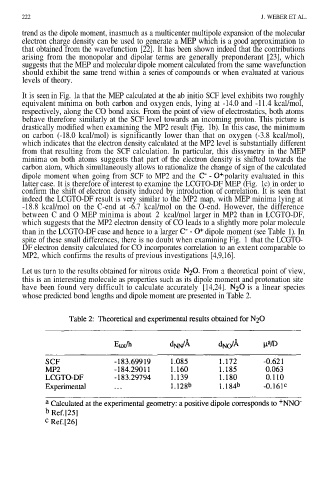
Let us turn to the results obtained for nitrous oxide From a theoretical point of view,
this is an interesting molecule as properties such as its dipole moment and protonation site
have been found very difficult to calculate accurately [14,24]. is a linear species
whose predicted bond lengths and dipole moment are presented in Table 2.