Page 291 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 291
274 M. TADJEDDINE AND J. P. FLAMENT
Applications to He and will give a deeper understanding of the determination of
the polarization functions.
3.3. APPLICATION TO He AND
Owing to their simplicity, the helium atom and the dihydrogen molecule have been
the object of experiments (Ref. 31 for of He; Ref. 32 for of ) and
calculations, some of them near the Hartree–Fock limit (Ref. 33 for He and Ref.
34–36 for ). In order to test our polarization functions, we have taken the zeroth
order basis set from the literature so as to describe the system best and our
references values are the HF limit for any observable
3.3.1. Helium
The field–free atom has the configuration the unperturbed wavefunction is de-
scribed through a Huzinaga’s CGTOs set (the 10 CGTOs one in Ref. 37). We use
the {2p, 3p} orbitals for and {3s/3d,4s/4d,5s/5d} orbitals for The
polarizability has been maximized with respect to the exponent of the STOs of
by using these polarization functions only; we have obtained : Then
this value has been given also to the exponent of the STOs in at the second
step of our calculations for the computation of
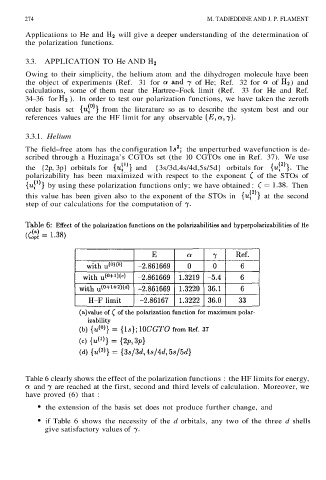
Table 6 clearly shows the effect of the polarization functions : the HF limits for energy,
and are reached at the first, second and third levels of calculation. Moreover, we
have proved (6) that :
• the extension of the basis set does not produce further change, and
• if Table 6 shows the necessity of the d orbitals, any two of the three d shells
give satisfactory values of