Page 63 - Sustainable On-Site CHP Systems Design, Construction, and Operations
P. 63
42 CHP B a s i c s
a shortage of oxygen results in the formation of carbon monoxide in the exhaust gases
(incomplete combustion).
Turbo- or supercharged engines can burn a mixture of fuel and air which has more air.
This fuel-air mixture can result in both more energy from the fuel and a cleaner exhaust.
In the past, natural gas engines were operated at an air-fuel ratio that provided the
most horsepower for the air consumed. This method of operating an engine is termed
fuel rich (or rich burn), because of the “lambda” or proportion of the operating air-fuel
ratio to the chemically correct air-fuel ratio (see below) where all the fuel and oxygen is
consumed during combustion is less than 1. The inverse of the fuel-air ratio is the air-
fuel ratio. The chemically correct air-fuel ratio for complete combustion is also known
as the stoichiometric air-fuel ratio. The equivalence ratio is the ratio of the operating
air-fuel ratio to the stoichiometric air-fuel ratio:
λ = equivalence ratio = (operating air-fuel ratio)/(stoichiometric air-fuel ratio)
Currently, many natural gas engines are operated at a much leaner air-fuel mixture
to take advantage of lower emissions and fuel consumption characteristics. A leaner
air-fuel mixture (λ > 1.0) allows for a higher concentration of oxygen in the combustion
chamber, more than is required for combustion. Therefore, a higher concentration of
oxygen is present in the engine exhaust.
The combustion temperatures in natural gas engines are lower when operating at a
fuel-rich air-fuel ratio and rises as the λ approaches 1.0 (stoichiometric air-fuel ratio). As
the air-fuel ratio becomes leaner, the combustion temperature again decreases (see Fig. 7-1
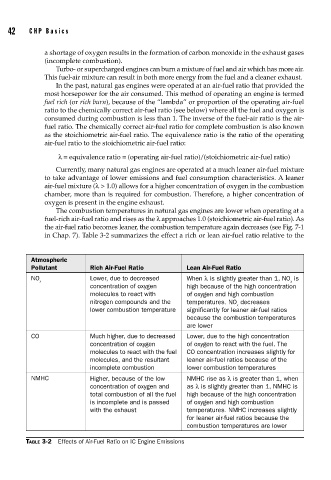
in Chap. 7). Table 3-2 summarizes the effect a rich or lean air-fuel ratio relative to the
Atmospheric
Pollutant Rich Air-Fuel Ratio Lean Air-Fuel Ratio
NO Lower, due to decreased When λ is slightly greater than 1, NO is
x x
concentration of oxygen high because of the high concentration
molecules to react with of oxygen and high combustion
nitrogen compounds and the temperatures. NO decreases
x
lower combustion temperature significantly for leaner air-fuel ratios
because the combustion temperatures
are lower
CO Much higher, due to decreased Lower, due to the high concentration
concentration of oxygen of oxygen to react with the fuel. The
molecules to react with the fuel CO concentration increases slightly for
molecules, and the resultant leaner air-fuel ratios because of the
incomplete combustion lower combustion temperatures
NMHC Higher, because of the low NMHC rise as λ is greater than 1, when
concentration of oxygen and as λ is slightly greater than 1, NMHC is
total combustion of all the fuel high because of the high concentration
is incomplete and is passed of oxygen and high combustion
with the exhaust temperatures. NMHC increases slightly
for leaner air-fuel ratios because the
combustion temperatures are lower
TABLE 3-2 Effects of Air-Fuel Ratio on IC Engine Emissions