Page 172 - Synthetic Fuels Handbook
P. 172
158 CHAPTER FIVE
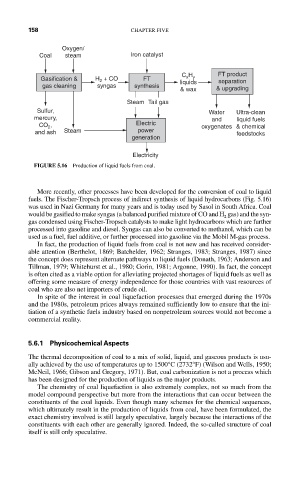
Oxygen/
Coal steam Iron catalyst
C H FT product
Gasification & H + CO FT x y separation
2
gas cleaning syngas synthesis liquids
& wax & upgrading
Steam Tail gas
Sulfur, Water Ultra-clean
mercury, and liquid fuels
CO , Electric oxygenates & chemical
2
and ash Steam power feedstocks
generation
Electricity
FIGURE 5.16 Production of liquid fuels from coal.
More recently, other processes have been developed for the conversion of coal to liquid
fuels. The Fischer-Tropsch process of indirect synthesis of liquid hydrocarbons (Fig. 5.16)
was used in Nazi Germany for many years and is today used by Sasol in South Africa. Coal
would be gasified to make syngas (a balanced purified mixture of CO and H gas) and the syn-
2
gas condensed using Fischer-Tropsch catalysts to make light hydrocarbons which are further
processed into gasoline and diesel. Syngas can also be converted to methanol, which can be
used as a fuel, fuel additive, or further processed into gasoline via the Mobil M-gas process.
In fact, the production of liquid fuels from coal is not new and has received consider-
able attention (Berthelot, 1869; Batchelder, 1962; Stranges, 1983; Stranges, 1987) since
the concept does represent alternate pathways to liquid fuels (Donath, 1963; Anderson and
Tillman, 1979; Whitehurst et al., 1980; Gorin, 1981; Argonne, 1990). In fact, the concept
is often cited as a viable option for alleviating projected shortages of liquid fuels as well as
offering some measure of energy independence for those countries with vast resources of
coal who are also net importers of crude oil.
In spite of the interest in coal liquefaction processes that emerged during the 1970s
and the 1980s, petroleum prices always remained sufficiently low to ensure that the ini-
tiation of a synthetic fuels industry based on nonpetroleum sources would not become a
commercial reality.
5.6.1 Physicochemical Aspects
The thermal decomposition of coal to a mix of solid, liquid, and gaseous products is usu-
ally achieved by the use of temperatures up to 1500°C (2732°F) (Wilson and Wells, 1950;
McNeil, 1966; Gibson and Gregory, 1971). But, coal carbonization is not a process which
has been designed for the production of liquids as the major products.
The chemistry of coal liquefaction is also extremely complex, not so much from the
model compound perspective but more from the interactions that can occur between the
constituents of the coal liquids. Even though many schemes for the chemical sequences,
which ultimately result in the production of liquids from coal, have been formulated, the
exact chemistry involved is still largely speculative, largely because the interactions of the
constituents with each other are generally ignored. Indeed, the so-called structure of coal
itself is still only speculative.