Page 259 - Synthetic Fuels Handbook
P. 259
FUELS FROM BIOMASS 245
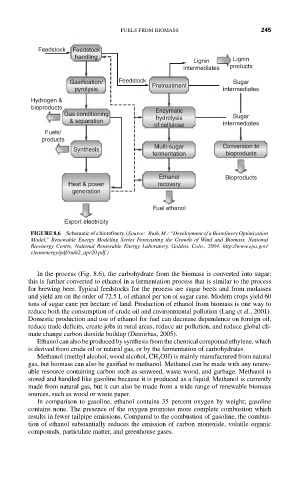
Feedstock Feedstock
handling
Lignin Lignin
intermediates products
Gasification/ Feedstock Pretreatment Sugar
pyrolysis intermediates
Hydrogen &
bioproducts
Gas conditioning Enzymatic Sugar
& separation hydrolysis intermediates
of cellulose
Fuels/
products
Multi-sugar Conversion to
Synthesis
fermentation bioproducts
Ethanol Bioproducts
Heat & power recovery
generation
Fuel ethanol
Export electricity
FIGURE 8.6 Schematic of a biorefinery. (Source: Ruth, M.: “Development of a Biorefinery Optimization
Model,” Renewable Energy Modeling Series Forecasting the Growth of Wind and Biomass, National
Bioenergy Centre, National Renewable Energy Laboratory, Golden, Colo., 2004, http://www.epa.gov/
cleanenergy/pdf/ruth2_apr20.pdf.)
In the process (Fig. 8.6), the carbohydrate from the biomass is converted into sugar;
this is further converted to ethanol in a fermentation process that is similar to the process
for brewing beer. Typical feedstocks for the process are sugar beets and from molasses
and yield are on the order of 72.5 L of ethanol per ton of sugar cane. Modern crops yield 60
tons of sugar cane per hectare of land. Production of ethanol from biomass is one way to
reduce both the consumption of crude oil and environmental pollution (Lang et al., 2001).
Domestic production and use of ethanol for fuel can decrease dependence on foreign oil,
reduce trade deficits, create jobs in rural areas, reduce air pollution, and reduce global cli-
mate change carbon dioxide buildup (Demirbas, 2005).
Ethanol can also be produced by synthesis from the chemical compound ethylene, which
is derived from crude oil or natural gas, or by the fermentation of carbohydrates.
Methanol (methyl alcohol, wood alcohol, CH OH) is mainly manufactured from natural
3
gas, but biomass can also be gasified to methanol. Methanol can be made with any renew-
able resource containing carbon such as seaweed, waste wood, and garbage. Methanol is
stored and handled like gasoline because it is produced as a liquid. Methanol is currently
made from natural gas, but it can also be made from a wide range of renewable biomass
sources, such as wood or waste paper.
In comparison to gasoline, ethanol contains 35 percent oxygen by weight; gasoline
contains none. The presence of the oxygen promotes more complete combustion which
results in fewer tailpipe emissions. Compared to the combustion of gasoline, the combus-
tion of ethanol substantially reduces the emission of carbon monoxide, volatile organic
compounds, particulate matter, and greenhouse gases.