Page 260 - Synthetic Fuels Handbook
P. 260
246 CHAPTER EIGHT
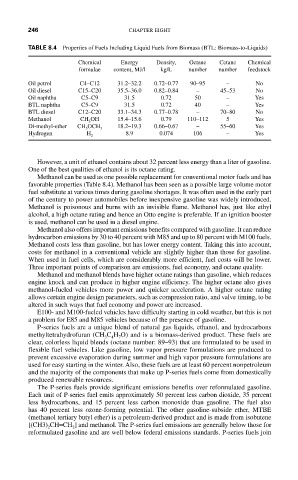
TABLE 8.4 Properties of Fuels Including Liquid Fuels from Biomass (BTL: Biomass-to-Liquids)
Chemical Energy Density, Octane Cetane Chemical
formulae content, MJ/l kg/L number number feedstock
Oil petrol C4–C12 31.2–32.2 0.72–0.77 90–95 – No
Oil diesel C15–C20 35.5–36.0 0.82–0.84 – 45–53 No
Oil naphtha C5–C9 31.5 0.72 50 – Yes
BTL naphtha C5–C9 31.5 0.72 40 – Yes
BTL diesel C12–C20 33.1–34.3 0.77–0.78 – 70–80 No
Methanol CH OH 15.4–15.6 0.79 110–112 5 Yes
3
Di-methyl-ether CH OCH 3 18.2–19.3 0.66–0.67 – 55–60 Yes
3
Hydrogen H 2 8.9 0.074 106 – Yes
However, a unit of ethanol contains about 32 percent less energy than a liter of gasoline.
One of the best qualities of ethanol is its octane rating.
Methanol can be used as one possible replacement for conventional motor fuels and has
favorable properties (Table 8.4). Methanol has been seen as a possible large volume motor
fuel substitute at various times during gasoline shortages. It was often used in the early part
of the century to power automobiles before inexpensive gasoline was widely introduced.
Methanol is poisonous and burns with an invisible flame. Methanol has, just like ethyl
alcohol, a high octane rating and hence an Otto engine is preferable. If an ignition booster
is used, methanol can be used in a diesel engine.
Methanol also offers important emissions benefits compared with gasoline. It can reduce
hydrocarbon emissions by 30 to 40 percent with M85 and up to 80 percent with M100 fuels.
Methanol costs less than gasoline, but has lower energy content. Taking this into account,
costs for methanol in a conventional vehicle are slightly higher than those for gasoline.
When used in fuel cells, which are considerably more efficient, fuel costs will be lower.
Three important points of comparison are emissions, fuel economy, and octane quality.
Methanol and methanol blends have higher octane ratings than gasoline, which reduces
engine knock and can produce in higher engine efficiency. The higher octane also gives
methanol-fueled vehicles more power and quicker acceleration. A higher octane rating
allows certain engine design parameters, such as compression ratio, and valve timing, to be
altered in such ways that fuel economy and power are increased.
E100- and M100-fueled vehicles have difficulty starting in cold weather, but this is not
a problem for E85 and M85 vehicles because of the presence of gasoline.
P-series fuels are a unique blend of natural gas liquids, ethanol, and hydrocarbons
methyltetrahydrofuran (CH C H O) and is a biomass-derived product. These fuels are
3
7
4
clear, colorless liquid blends (octane number: 89–93) that are formulated to be used in
flexible fuel vehicles. Like gasoline, low vapor pressure formulations are produced to
prevent excessive evaporation during summer and high vapor pressure formulations are
used for easy starting in the winter. Also, these fuels are at least 60 percent nonpetroleum
and the majority of the components that make up P-series fuels come from domestically
produced renewable resources.
The P-series fuels provide significant emissions benefits over reformulated gasoline.
Each unit of P-series fuel emits approximately 50 percent less carbon dioxide, 35 percent
less hydrocarbons, and 15 percent less carbon monoxide than gasoline. The fuel also
has 40 percent less ozone-forming potential. The other gasoline-subside ether, MTBE
(methanol tertiary butyl ether) is a petroleum-derived product and is made from isobutene
[(CH3) CH=CH ] and methanol. The P-series fuel emissions are generally below those for
2
2
reformulated gasoline and are well below federal emissions standards. P-series fuels join