Page 300 - Synthetic Fuels Handbook
P. 300
286 CHAPTER NINE
absence of a pretreatment step. These advantages favor the use of the acid-catalyzed pro-
cess when using waste cooking oil as the raw material (Zhang et al., 2003).
Enzyme (such as lipase)-catalyzed reactions have advantages over traditional chemical-
catalyzed reactions: the generation of no by-products, easy product recovery, mild reaction
conditions, and catalyst recycling. Also, enzymatic reactions are insensitive to free fatty acids
and water content in waste cooking oil (Kulkarni and Dalai, 2006). As for the enzyme-catalyzed
system, it requires a much longer reaction time than the other two systems (Zhang et al., 2003).
The enzyme reactions are highly specific and chemically clean. Because the alcohol can be
inhibitory to the enzyme, a typical strategy is to feed the alcohol into the reactor in three steps
of 1:1 mole ratio each. The reactions are very slow, with a three step sequence requiring from
4 to 40 hours, or more. The reaction conditions are modest, from 35 to 45°C (van
Gerpen et al., 2004). The main problem of the enzyme-catalyzed process is the high
cost of the lipases used as catalyst (Royon et al., 2007).
Synthesis of biodiesel using enzymes such as Candida antarctica, Candida rugasa,
Pseudomonas cepacia, immobilized lipase (Lipozyme RMIM), Pseudomonas spp., and
Rhizomucor miehei is well reported in the literature. In the previously mentioned work
of Shah and Gupta (2007), the best yield 98 percent (w/w) was obtained by using
P. cepacia lipase immobilized on celite at 50 C in the presence of 4 to 5 percent (w/w)
water in 8 hours.
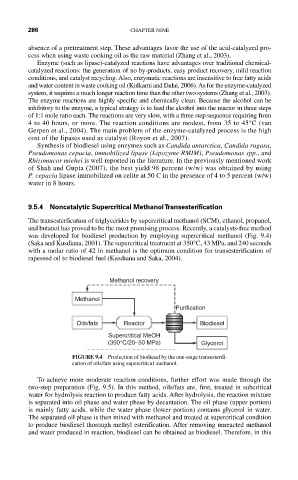
9.5.4 Noncatalytic Supercritical Methanol Transesterification
The transesterfication of triglycerides by supercritical methanol (SCM), ethanol, propanol,
and butanol has proved to be the most promising process. Recently, a catalysts-free method
was developed for biodiesel production by employing supercritical methanol (Fig. 9.4)
(Saka and Kusdiana, 2001). The supercritical treatment at 350°C, 43 MPa, and 240 seconds
with a molar ratio of 42 in methanol is the optimum condition for transesterification of
rapeseed oil to biodiesel fuel (Kusdiana and Saka, 2004).
Methanol recovery
Methanol
Purification
Oils/fats Reactor Biodiesel
Supercritical MeOH
(350°C/20–50 MPa) Glycerol
FIGURE 9.4 Production of biodiesel by the one-stage transesterifi-
cation of oils/fats using supercritical methanol.
To achieve more moderate reaction conditions, further effort was made through the
two-step preparation (Fig. 9.5). In this method, oils/fats are, first, treated in subcritical
water for hydrolysis reaction to produce fatty acids. After hydrolysis, the reaction mixture
is separated into oil phase and water phase by decantation. The oil phase (upper portion)
is mainly fatty acids, while the water phase (lower portion) contains glycerol in water.
The separated oil phase is then mixed with methanol and treated at supercritical condition
to produce biodiesel thorough methyl esterification. After removing unreacted methanol
and water produced in reaction, biodiesel can be obtained as biodiesel. Therefore, in this