Page 301 - Synthetic Fuels Handbook
P. 301
FUELS FROM CROPS 287
MeOH recovery
Water MeOH
Purification
Oil phase
Oils/fats Reactor 1 Reactor 2
Subcritical water Subcritical MeOH
(270°C/7 MPa) (270°C/7 MPa)
Biodiesel
Waste
water
Water phase
Glycerol
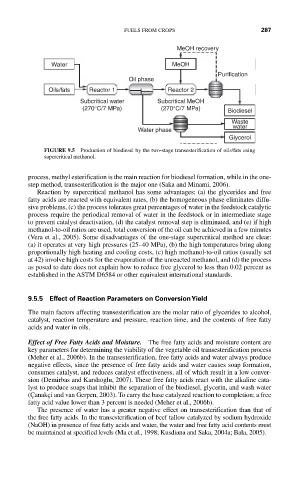
FIGURE 9.5 Production of biodiesel by the two-stage transesterification of oils/fats using
supercritical methanol.
process, methyl esterification is the main reaction for biodiesel formation, while in the one-
step method, transesterification is the major one (Saka and Minami, 2006).
Reaction by supercritical methanol has some advantages: (a) the glycerides and free
fatty acids are reacted with equivalent rates, (b) the homogeneous phase eliminates diffu-
sive problems, (c) the process tolerates great percentages of water in the feedstock catalytic
process require the periodical removal of water in the feedstock or in intermediate stage
to prevent catalyst deactivation, (d) the catalyst removal step is eliminated, and (e) if high
methanol-to-oil ratios are used, total conversion of the oil can be achieved in a few minutes
(Vera et al., 2005). Some disadvantages of the one-stage supercritical method are clear:
(a) it operates at very high pressures (25–40 MPa), (b) the high temperatures bring along
proportionally high heating and cooling costs, (c) high methanol-to-oil ratios (usually set
at 42) involve high costs for the evaporation of the unreacted methanol, and (d) the process
as posed to date does not explain how to reduce free glycerol to less than 0.02 percent as
established in the ASTM D6584 or other equivalent international standards.
9.5.5 Effect of Reaction Parameters on Conversion Yield
The main factors affecting transesterification are the molar ratio of glycerides to alcohol,
catalyst, reaction temperature and pressure, reaction time, and the contents of free fatty
acids and water in oils.
Effect of Free Fatty Acids and Moisture. The free fatty acids and moisture content are
key parameters for determining the viability of the vegetable oil transesterification process
(Meher et al., 2006b). In the transesterification, free fatty acids and water always produce
negative effects, since the presence of free fatty acids and water causes soap formation,
consumes catalyst, and reduces catalyst effectiveness, all of which result in a low conver-
sion (Demirbas and Karslıoglu, 2007). These free fatty acids react with the alkaline cata-
lyst to produce soaps that inhibit the separation of the biodiesel, glycerin, and wash water
(Çanakçi and van Gerpen, 2003). To carry the base catalyzed reaction to completion; a free
fatty acid value lower than 3 percent is needed (Meher et al., 2006b).
The presence of water has a greater negative effect on transesterification than that of
the free fatty acids. In the transesterification of beef tallow catalyzed by sodium hydroxide
(NaOH) in presence of free fatty acids and water, the water and free fatty acid contents must
be maintained at specified levels (Ma et al., 1998; Kusdiana and Saka, 2004a; Bala, 2005).