Page 483 - Tandem Techniques
P. 483
Page 468
12.8). It follows that reference spectra that are to be used for solute identification should also be
obtained from the TLC plate in the same manner. The mass of solute in each spot examined was about
10 µg but it was estimated that about 1 µg would be sufficient for a recognizable spectrum to be
obtained.
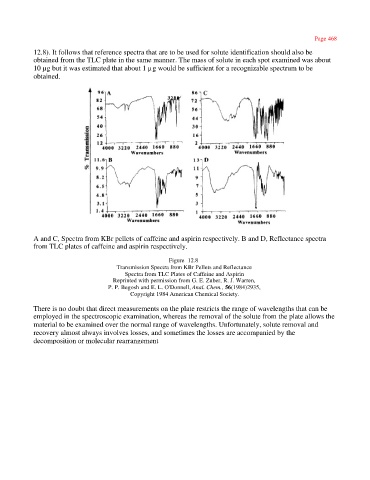
A and C, Spectra from KBr pellets of caffeine and aspirin respectively. B and D, Reflectance spectra
from TLC plates of caffeine and aspirin respectively.
Figure 12.8
Transmission Spectra from KBr Pellets and Reflectance
Spectra from TLC Plates of Caffeine and Aspirin
Reprinted with permission from G. E. Zuber, R. J. Warren,
P. P. Begosh and E. L. O'Donnell, Anal. Chem., 56(1984)2935,
Copyright 1984 American Chemical Society.
There is no doubt that direct measurements on the plate restricts the range of wavelengths that can be
employed in the spectroscopic examination, whereas the removal of the solute from the plate allows the
material to be examined over the normal range of wavelengths. Unfortunately, solute removal and
recovery almost always involves losses, and sometimes the losses are accompanied by the
decomposition or molecular rearrangement