Page 145 - The Biochemistry of Inorganic Polyphosphates
P. 145
20:32
March 9, 2004
Char Count= 0
WU095-08
WU095/Kulaev
Escherichia coli 129
1
35
% of initial activity (× 10 3 ) 25 1000 2 3
15
600
5 200
4 5
120 360 120 240 120 240 120 240 120 360
Time (min)
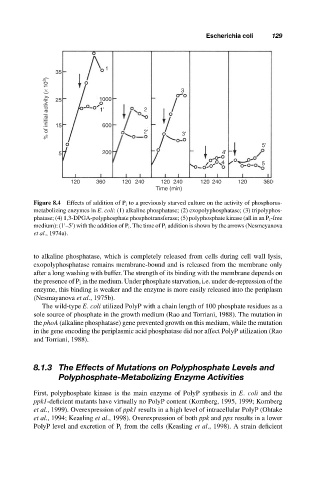
Figure 8.4 Effects of addition of P i to a previously starved culture on the activity of phosphorus-
metabolizing enzymes in E. coli: (1) alkaline phosphatase; (2) exopolyphosphatase; (3) tripolyphos-
phatase; (4) 1,3-DPGA-polyphosphate phosphotransferase; (5) polyphosphate kinase (all in an P i -free
medium): (1 –5 ) with the addition of P i . The time of P i addition is shown by the arrows (Nesmeyanova
et al., 1974a).
to alkaline phosphatase, which is completely released from cells during cell wall lysis,
exopolyphosphatase remains membrane-bound and is released from the membrane only
after a long washing with buffer. The strength of its binding with the membrane depends on
the presence of P i in the medium. Under phosphate starvation, i.e. under de-repression of the
enzyme, this binding is weaker and the enzyme is more easily released into the periplasm
(Nesmayanova et al., 1975b).
The wild-type E. coli utilized PolyP with a chain length of 100 phosphate residues as a
sole source of phosphate in the growth medium (Rao and Torriani, 1988). The mutation in
the phoA (alkaline phosphatase) gene prevented growth on this medium, while the mutation
in the gene encoding the periplasmic acid phosphatase did nor affect PolyP utilization (Rao
and Torriani, 1988).
8.1.3 The Effects of Mutations on Polyphosphate Levels and
Polyphosphate-Metabolizing Enzyme Activities
First, polyphosphate kinase is the main enzyme of PolyP synthesis in E. coli and the
ppk1-deficient mutants have virtually no PolyP content (Kornberg, 1995, 1999; Kornberg
et al., 1999). Overexpression of ppk1 results in a high level of intracellular PolyP (Ohtake
et al., 1994; Keasling et al., 1998). Overexpression of both ppk and ppx results in a lower
PolyP level and excretion of P i from the cells (Keasling et al., 1998). A strain deficient