Page 144 - The Biochemistry of Inorganic Polyphosphates
P. 144
WU095/Kulaev
WU095-08
Peculiarities of polyphosphate metabolism
128 March 9, 2004 20:32 Char Count= 0
(a) (b)
APase (× 10 3 )
PPase 40
30 1 2
% of initial activity 1000 20 3 PP kinase and 1,3-DPGA-PP phosphotransferase
700
10
400
200
3 2
5
100 5 100
4 4 1
60 120 180 300 60 180 300
Time (min) Time (min)
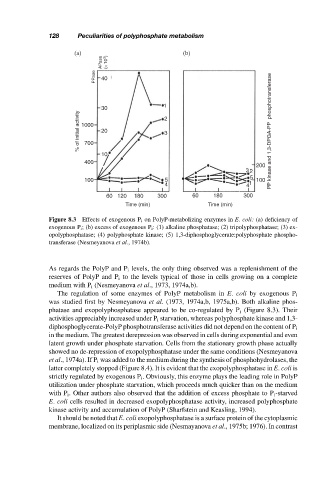
Figure 8.3 Effects of exogenous P i on PolyP-metabolizing enzymes in E. coli: (a) deficiency of
exogenous P i ; (b) excess of exogenous P i : (1) alkaline phosphatase; (2) tripolyphosphatase; (3) ex-
opolyphosphatase; (4) polyphosphate kinase; (5) 1,3-diphosphoglycerate:polyphosphate phospho-
transferase (Nesmeyanova et al., 1974b).
As regards the PolyP and P i levels, the only thing observed was a replenishment of the
reserves of PolyP and P i to the levels typical of those in cells growing on a complete
medium with P i (Nesmeyanova et al., 1973, 1974a,b).
The regulation of some enzymes of PolyP metabolism in E. coli by exogenous P i
was studied first by Nesmeyanova et al. (1973, 1974a,b, 1975a,b). Both alkaline phos-
phatase and exopolyphosphatase appeared to be co-regulated by P i (Figure 8.3). Their
activities appreciably increased under P i starvation, whereas polyphosphate kinase and 1,3-
diphosphoglycerate-PolyP phosphotransferase activities did not depend on the content of P i
in the medium. The greatest derepression was observed in cells during exponential and even
latent growth under phosphate starvation. Cells from the stationary growth phase actually
showed no de-repression of exopolyphosphatase under the same conditions (Nesmeyanova
et al., 1974a). If P i was added to the medium during the synthesis of phosphohydrolases, the
latter completely stopped (Figure 8.4). It is evident that the exopolyphosphatase in E. coli is
strictly regulated by exogenous P i . Obviously, this enzyme plays the leading role in PolyP
utilization under phosphate starvation, which proceeds much quicker than on the medium
with P i . Other authors also observed that the addition of excess phosphate to P i -starved
E. coli cells resulted in decreased exopolyphosphatase activity, increased polyphosphate
kinase activity and accumulation of PolyP (Sharfstein and Keasling, 1994).
It should be noted that E. coli exopolyphosphatase is a surface protein of the cytoplasmic
membrane, localized on its periplasmic side (Nesmayanova et al., 1975b; 1976). In contrast