Page 20 - The Biochemistry of Inorganic Polyphosphates
P. 20
WU095/Kulaev
WU095-O1
Chemical structures and properties of inorganic phosphates
4 March 9, 2004 15:21 Char Count= 0
data which shed much light on the structures and properties of this group of compounds,
threw into perhaps even greater confusion both the chemical basis of the nomenclature
of these compounds, and the names of the compounds themselves. This is perhaps hardly
surprising, since these investigations were carried out with compounds of inadequate purity,
using rather crude investigation methods. It was thanks to the work of Thilo (1950, 1955,
1956, 1959, 1962), Van Wazer (1950, 1958), Ebel (1951, 1952a–d, 1953a,b) and Boulle
(1965) that the chemical structures and properties of this group of compounds were finally
established, thus making it possible to bring order into their classification (Van Wazer and
Griffith, 1955; Thilo and Sonntag, 1957).
Accordingtothecurrentclassification,condensedphosphatesaredividedintocyclophos-
phates, polyphosphates and branched inorganic phosphates (or ‘ultraphosphates’).
1.1.1 Cyclophosphates
The true cyclophosphates (metaphosphates) have the composition which, since the time
of Graham, has been incorrectly assigned to the whole group of condensed phosphates,
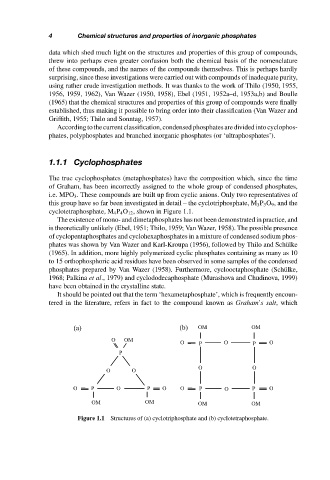
i.e. MPO 3 . These compounds are built up from cyclic anions. Only two representatives of
this group have so far been investigated in detail – the cyclotriphosphate, M 3 P 3 O 9 , and the
cyclotetraphosphate, M 4 P 4 O 12 , shown in Figure 1.1.
The existence of mono- and dimetaphosphates has not been demonstrated in practice, and
is theoretically unlikely (Ebel, 1951; Thilo, 1959; Van Wazer, 1958). The possible presence
of cyclopentaphosphates and cyclohexaphosphates in a mixture of condensed sodium phos-
phates was shown by Van Wazer and Karl-Kroupa (1956), followed by Thilo and Sch¨ulke
(1965). In addition, more highly polymerized cyclic phosphates containing as many as 10
to 15 orthophosphoric acid residues have been observed in some samples of the condensed
phosphates prepared by Van Wazer (1958). Furthermore, cyclooctaphosphate (Sch¨ulke,
1968; Palkina et al., 1979) and cyclododecaphosphate (Murashova and Chudinova, 1999)
have been obtained in the crystalline state.
It should be pointed out that the term ‘hexametaphosphate’, which is frequently encoun-
tered in the literature, refers in fact to the compound known as Graham’s salt, which
(a) (b) OM OM
O OM
O P O P O
P
O O
O O
O P O P O O P O P O
OM OM OM OM
Figure 1.1 Structures of (a) cyclotriphosphate and (b) cyclotetraphosphate.