Page 22 - The Biochemistry of Inorganic Polyphosphates
P. 22
WU095/Kulaev
WU095-O1
Chemical structures and properties of inorganic phosphates
6 March 9, 2004 15:21 Char Count= 0
100
a
80 b
Total P2O5 (%) 60
40
n = 193 c d
20
0 100 200 300 400 500 600
n
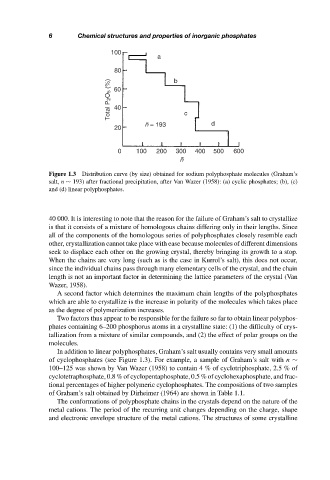
Figure 1.3 Distribution curve (by size) obtained for sodium polyphosphate molecules (Graham’s
salt, n ∼ 193) after fractional precipitation, after Van Wazer (1958): (a) cyclic phosphates; (b), (c)
and (d) linear polyphosphates.
40 000. It is interesting to note that the reason for the failure of Graham’s salt to crystallize
is that it consists of a mixture of homologous chains differing only in their lengths. Since
all of the components of the homologous series of polyphosphates closely resemble each
other, crystallization cannot take place with ease because molecules of different dimensions
seek to displace each other on the growing crystal, thereby bringing its growth to a stop.
When the chains are very long (such as is the case in Kurrol’s salt), this does not occur,
since the individual chains pass through many elementary cells of the crystal, and the chain
length is not an important factor in determining the lattice parameters of the crystal (Van
Wazer, 1958).
A second factor which determines the maximum chain lengths of the polyphosphates
which are able to crystallize is the increase in polarity of the molecules which takes place
as the degree of polymerization increases.
Two factors thus appear to be responsible for the failure so far to obtain linear polyphos-
phates containing 6–200 phosphorus atoms in a crystalline state: (1) the difficulty of crys-
tallization from a mixture of similar compounds, and (2) the effect of polar groups on the
molecules.
In addition to linear polyphosphates, Graham’s salt usually contains very small amounts
of cyclophosphates (see Figure 1.3). For example, a sample of Graham’s salt with n ∼
100–125 was shown by Van Wazer (1958) to contain 4 % of cyclotriphosphate, 2.5 % of
cyclotetraphosphate, 0.8 % of cyclopentaphosphate, 0.5 % of cyclohexaphosphate, and frac-
tional percentages of higher polymeric cyclophosphates. The compositions of two samples
of Graham’s salt obtained by Dirheimer (1964) are shown in Table 1.1.
The conformations of polyphosphate chains in the crystals depend on the nature of the
metal cations. The period of the recurring unit changes depending on the charge, shape
and electronic envelope structure of the metal cations. The structures of some crystalline