Page 27 - The Biochemistry of Inorganic Polyphosphates
P. 27
March 9, 2004
WU095/Kulaev
15:21
WU095-O1
Char Count= 0
Some chemical properties of condensed inorganic polyphosphates 11
The branching points in branched phosphates, in which one atom is bonded through
oxygen to three other phosphorus atoms, are extremely labile. The rate of hydrolysis of
the branching points in the reticular phosphates in aqueous solution at 25 C, resulting in
◦
the formation of linear polyphosphates, is about 1000 times greater than that of the P–O–P
bonds in the linear polyphosphates. Hydrolysis of the branching points liberates 28 kcal
mol −1 (Van Wazer, 1958), which is much more than that liberated in the hydrolysis of the
‘central’ phosphoric anhydride bonds.
The linear polyphosphates and cyclophosphates are hydrolysed extremely slowly at
neutral pH and room temperature in comparison with other polyacids such as polyarsenates
and polyvanadates, and are unique in this respect. The ‘half-hydrolysis time’ for the P–O–P
bonds in linear polyphosphates at pH 7 and 25 C is several years (Van Wazer, 1958). The
◦
rate of hydrolysis of these bonds is increased by raising the temperature, reducing the pH,
and by the presence in the solution of colloidal gels and complex cations. The hydrolysis
of these bonds is dependent on the ionic strength of the solutions (Van Wazer, 1958).
◦
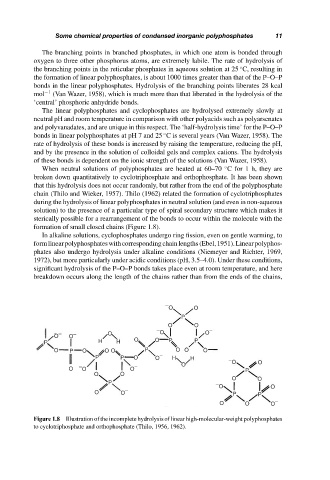
When neutral solutions of polyphosphates are heated at 60–70 C for 1 h, they are
broken down quantitatively to cyclotriphosphate and orthophosphate. It has been shown
that this hydrolysis does not occur randomly, but rather from the end of the polyphosphate
chain (Thilo and Wieker, 1957). Thilo (1962) related the formation of cyclotriphosphates
during the hydrolysis of linear polyphosphates in neutral solution (and even in non-aqueous
solution) to the presence of a particular type of spiral secondary structure which makes it
sterically possible for a rearrangement of the bonds to occur within the molecule with the
formation of small closed chains (Figure 1.8).
In alkaline solutions, cyclophosphates undergo ring fission, even on gentle warming, to
formlinearpolyphosphateswithcorrespondingchainlengths(Ebel,1951).Linearpolyphos-
phates also undergo hydrolysis under alkaline conditions (Niemeyer and Richter, 1969,
1972), but more particularly under acidic conditions (pH, 3.5–4.0). Under these conditions,
significant hydrolysis of the P–O–P bonds takes place even at room temperature, and here
breakdown occurs along the length of the chains rather than from the ends of the chains,
−
O O
P
O O
− −
− − O O O
O O
P H H O O P P
O P O O O P O O O
P P O O − H H −
− − O O O
O O O P
O O
O O
P −
− O O
O O P P
−
O O O
Figure 1.8 Illustration of the incomplete hydrolysis of linear high-molecular-weight polyphosphates
to cyclotriphosphate and orthophosphate (Thilo, 1956, 1962).