Page 28 - The Biochemistry of Inorganic Polyphosphates
P. 28
WU095/Kulaev
WU095-O1
Chemical structures and properties of inorganic phosphates
12 March 9, 2004 15:21 Char Count= 0
100
g
Monomer
80 f Intermediate
Total P 2 O 5 (%) 60 a b c e d chains
40
Rings
20
0 0.5 1.0 1.5 2.0 2.5 3.0
Time (h)
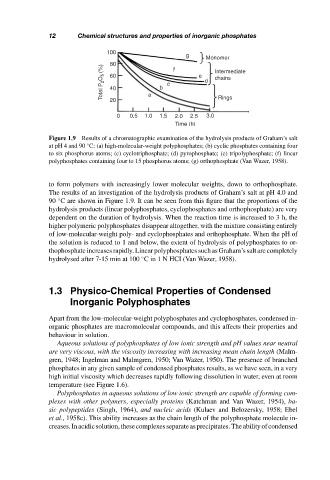
Figure 1.9 Results of a chromatographic examination of the hydrolysis products of Graham’s salt
at pH 4 and 90 C: (a) high-molecular-weight polyphosphates; (b) cyclic phosphates containing four
◦
to six phosphorus atoms; (c) cyclotriphosphate; (d) pyrophosphate; (e) tripolyphosphate; (f) linear
polyphosphates containing four to 15 phosphorus atoms; (g) orthophosphate (Van Wazer, 1958).
to form polymers with increasingly lower molecular weights, down to orthophosphate.
The results of an investigation of the hydrolysis products of Graham’s salt at pH 4.0 and
90 C are shown in Figure 1.9. It can be seen from this figure that the proportions of the
◦
hydrolysis products (linear polyphosphates, cyclophosphates and orthophosphate) are very
dependent on the duration of hydrolysis. When the reaction time is increased to 3 h, the
higher polymeric polyphosphates disappear altogether, with the mixture consisting entirely
of low-molecular-weight poly- and cyclophosphates and orthophosphate. When the pH of
the solution is reduced to 1 and below, the extent of hydrolysis of polyphosphates to or-
thophosphate increases rapidly. Linear polyphosphates such as Graham’s salt are completely
hydrolysed after 7-15 min at 100 C in 1 N HCI (Van Wazer, 1958).
◦
1.3 Physico-Chemical Properties of Condensed
Inorganic Polyphosphates
Apart from the low-molecular-weight polyphosphates and cyclophosphates, condensed in-
organic phosphates are macromolecular compounds, and this affects their properties and
behaviour in solution.
Aqueous solutions of polyphosphates of low ionic strength and pH values near neutral
are very viscous, with the viscosity increasing with increasing mean chain length (Malm-
gren, 1948; Ingelman and Malmgren, 1950; Van Wazer, 1950). The presence of branched
phosphates in any given sample of condensed phosphates results, as we have seen, in a very
high initial viscosity which decreases rapidly following dissolution in water, even at room
temperature (see Figure 1.6).
Polyphosphates in aqueous solutions of low ionic strength are capable of forming com-
plexes with other polymers, especially proteins (Katchman and Van Wazer, 1954), ba-
sic polypeptides (Singh, 1964), and nucleic acids (Kulaev and Belozersky, 1958; Ebel
et al., 1958c). This ability increases as the chain length of the polyphosphate molecule in-
creases.Inacidicsolution,thesecomplexesseparateasprecipitates.Theabilityofcondensed