Page 25 - The Biochemistry of Inorganic Polyphosphates
P. 25
15:21
March 9, 2004
Char Count= 0
WU095-O1
WU095/Kulaev
Some chemical properties of condensed inorganic polyphosphates 9
O O O
. . . P O P O P O . . .
OM O OM
O P O
O
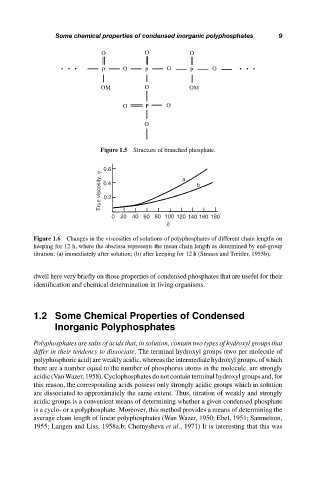
Figure 1.5 Structure of branched phosphate.
0.6
a
0.4 b
0.2
0 20 40 60 80 100 120 140 160 180
n
Figure 1.6 Changes in the viscosities of solutions of polyphosphates of different chain lengths on
keeping for 12 h, where the abscissa represents the mean chain length as determined by end-group
titration: (a) immediately after solution; (b) after keeping for 12 h (Strauss and Treitler, 1955b).
dwell here very briefly on those properties of condensed phosphates that are useful for their
identification and chemical determination in living organisms.
1.2 Some Chemical Properties of Condensed
Inorganic Polyphosphates
Polyphosphates are salts of acids that, in solution, contain two types of hydroxyl groups that
differ in their tendency to dissociate. The terminal hydroxyl groups (two per molecule of
polyphosphoric acid) are weakly acidic, whereas the intermediate hydroxyl groups, of which
there are a number equal to the number of phosphorus atoms in the molecule, are strongly
acidic (Van Wazer, 1958). Cyclophosphates do not contain terminal hydroxyl groups and, for
this reason, the corresponding acids possess only strongly acidic groups which in solution
are dissociated to approximately the same extent. Thus, titration of weakly and strongly
acidic groups is a convenient means of determining whether a given condensed phosphate
is a cyclo- or a polyphosphate. Moreover, this method provides a means of determining the
average chain length of linear polyphosphates (Wan Wazer, 1950; Ebel, 1951; Samuelson,
1955; Langen and Liss, 1958a,b; Chernysheva et al., 1971) It is interesting that this was