Page 26 - The Biochemistry of Inorganic Polyphosphates
P. 26
WU095/Kulaev
WU095-O1
Chemical structures and properties of inorganic phosphates
10 March 9, 2004 15:21 Char Count= 0
the method used by Samuelson (1955) in showing for the first time that Graham’s salt was
not a cyclophosphate – as had been believed for almost 100 years – but a mixture of linear
polyphosphates.
All alkali metal salts of condensed polyphosphoric acids are soluble in water. Potassium
pyrophosphate is especially soluble, with, for example, 100 g of water dissolving 187.4 g
of K 4 P 2 O 7 at 25 C,207gat50 C, and 240 g at 75 C. Exceptions to this rule are the
◦
◦
◦
water-insoluble Kurrol’s salt (a macromolecular crystalline potassium polyphosphate), and
the compounds known as Maddrell’s salts (crystalline sodium polyphosphates of very high
molecular weight). Kurrol’s salt is readily soluble in dilute solutions of salts containing
+
cations of univalent metals (but not K ), for example, 0.2 M NaCl. It is worth mentioning
that Graham’s salt dissolves in water only when it is stirred rapidly. Without stirring, the
2+
compound forms a glue-like mass in water. Polyphosphates of divalent metals such as Ba ,
Pb 2+ and Mg 2+ are either completely insoluble or dissolve to only a very limited extent
in aqueous solutions. The polyphosphates of certain organic bases such as guanidine are
also sparingly soluble in water (Singh, 1964). Other solvents (liquid ammonia, anhydrous
formic acid, and organic solvents such as ethanol and acetone) dissolve only trace amounts
of sodium and ammonium polyphosphates. Low-molecular-weight polyphosphates dissolve
readily in very dilute aqueous alcoholic solutions, but addition of alcohol to these solutions
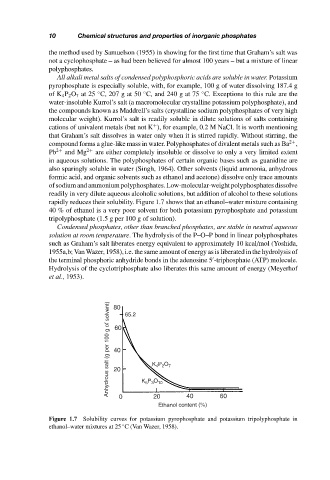
rapidly reduces their solubility. Figure 1.7 shows that an ethanol–water mixture containing
40 % of ethanol is a very poor solvent for both potassium pyrophosphate and potassium
tripolyphosphate (1.5 g per 100 g of solution).
Condensed phosphates, other than branched phosphates, are stable in neutral aqueous
solution at room temperature. The hydrolysis of the P–O–P bond in linear polyphosphates
such as Graham’s salt liberates energy equivalent to approximately 10 kcal/mol (Yoshida,
1955a,b; Van Wazer, 1958), i.e. the same amount of energy as is liberated in the hydrolysis of
the terminal phosphoric anhydride bonds in the adenosine 5 -triphosphate (ATP) molecule.
Hydrolysis of the cyclotriphosphate also liberates this same amount of energy (Meyerhof
et al., 1953). 80
Anhydrous salt (g per 100 g of solvent) 40 65.2 K P O 7
60
4 2
20
K P O
10
5 3
40
0
20
Ethanol content (%) 60
Figure 1.7 Solubility curves for potassium pyrophosphate and potassium tripolyphosphate in
ethanol–water mixtures at 25 C (Van Wazer, 1958).
◦