Page 48 - The Biochemistry of Inorganic Polyphosphates
P. 48
WU095/Kulaev
WU095-02
Methods of polyphosphate assay
32 March 9, 2004 15:25 Char Count= 0
(a)
Chemical shift, δ (ppm)
(b)
Chemical shift, δ (ppm)
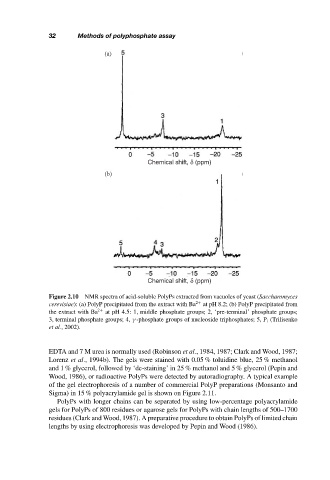
Figure 2.10 NMR spectra of acid-soluble PolyPs extracted from vacuoles of yeast (Saccharomyces
cerevisiae): (a) PolyP precipitated from the extract with Ba 2+ at pH 8.2; (b) PolyP precipitated from
the extract with Ba 2+ at pH 4.5: 1, middle phosphate groups; 2, ‘pre-terminal’ phosphate groups;
3, terminal phosphate groups; 4, γ -phosphate groups of nucleoside triphosphates; 5, P i (Trilisenko
et al., 2002).
EDTA and 7 M urea is normally used (Robinson et al., 1984, 1987; Clark and Wood, 1987;
Lorenz et al., 1994b). The gels were stained with 0.05 % toluidine blue, 25 % methanol
and 1 % glycerol, followed by ‘de-staining’ in 25 % methanol and 5 % glycerol (Pepin and
Wood, 1986), or radioactive PolyPs were detected by autoradiography. A typical example
of the gel electrophoresis of a number of commercial PolyP preparations (Monsanto and
Sigma) in 15 % polyacrylamide gel is shown on Figure 2.11.
PolyPs with longer chains can be separated by using low-percentage polyacrylamide
gels for PolyPs of 800 residues or agarose gels for PolyPs with chain lengths of 500–1700
residues (Clark and Wood, 1987). A preparative procedure to obtain PolyPs of limited chain
lengths by using electrophoresis was developed by Pepin and Wood (1986).