Page 115 - The engineering of chemical reactions
P. 115
The l/r Plot 99
positive order kinetics, and the difference becomes larger as the order of the reaction
increases. For zeroth order kinetics the sizes required for a given conversion are exactly
equal, and for negative order kinetics the CSTR requires a smaller volume than a PFTR
(or batch reactor). We will show later that this is not necessarily true in a nonisothermal
reactor, where the CSTR can “win” over the PFTR in both simplicity and residence time.
THE l/r PLOT
There is a graphical construction that shows the difference of residence times in different
types and combinations of chemical reactors. We write the mass balance equations as
CAo - CA
TCSTR = = &(CAo - CA)
r(cA)
and
CA
1
TPlTR = - ~ dCA
s r(cA)
CA0
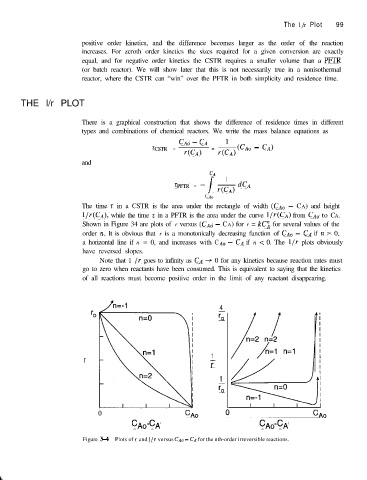
The time t in a CSTR is the area under the rectangle of width (CAM - CA) and height
l/r(CA), while the time t in a PFTR is the area under the curve l/r(CA) from CA,, to CA.
Shown in Figure 34 are plots of r versus (C,J~ - CA) for r = kC2 for several values of the
order n. It is obvious that r is a monotonically decreasing function of CA,, - CA if IZ > 0,
a horizontal line if n = 0, and increases with CA0 - CA if n < 0. The l/r plots obviously
have reversed slopes.
Note that 1 /r goes to infinity as CA + 0 for any kinetics because reaction rates must
go to zero when reactants have been consumed. This is equivalent to saying that the kinetics
of all reactions must become positive order in the limit of any reactant disappearing.
4
To
1 //;/ n=l
n=2
r
r
1
ro
0 cAo
CAo’CA CA&A
Figure 3-4 Plots of I and l/r versus CA,, - C, for the nth-order irreversible reactions.