Page 54 - The engineering of chemical reactions
P. 54
38 Reaction Rates, the Batch Reactor, and the Real World
We will write all reactor mass and heat balances as
accumulation = flow in - flow out + generation by reaction
an expression we will see many times in mass and energy balances throughout this book.
We remark before proceeding that equations such as
dC.4 = -kCA
-
. dt
and its integrated form
CA = cAoeekt
arise from a very special situation requiring both a single$rst-order irreversible reaction
and a constant-volume isothermal batch reactor. This example is almost trivial, although
we will use it frequently as a comparison with more interesting and accurate examples. The
assumption of first-order kinetics is a simple first guess for kinetics and a good starting
point before more elaborate calculations.
We note before proceeding that we must formulate and solve many mass-balance
equations. We strongly encourage the student not to memorize anything except the basic
defining relations. We stress that you should be able to derive every equation from these
definitions as needed. This is because (1) only by being able to do this will you understand
the principles of the subject: and (2) we need to make many different approximations, and
remembering the wrong equation is disastrous.
THE BATCH REACTOR
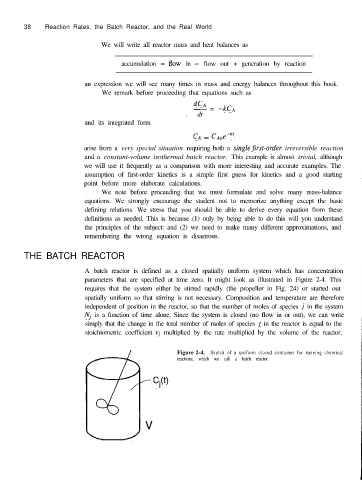
A batch reactor is defined as a closed spatially uniform system which has concentration
parameters that are specified at time zero. It might look as illustrated in Figure 2-4. This
requires that the system either be stirred rapidly (the propeller in Fig. 24) or started out
spatially uniform so that stirring is not necessary. Composition and temperature are therefore
independent of position in the reactor, so that the number of moles of species j in the system
Nj is a function of time alone, Since the system is closed (no flow in or out), we can write
simply that the change in the total number of moles of species j in the reactor is equal to the
stoichiometric coefficient vi multiplied by the rate multiplied by the volume of the reactor,
Figure 2-4. Sketch of a uniform closed container for running chemical
reactions, which we call a batch reactor.
cj(t)