Page 57 - The engineering of chemical reactions
P. 57
The Batch Reactor 41
kt
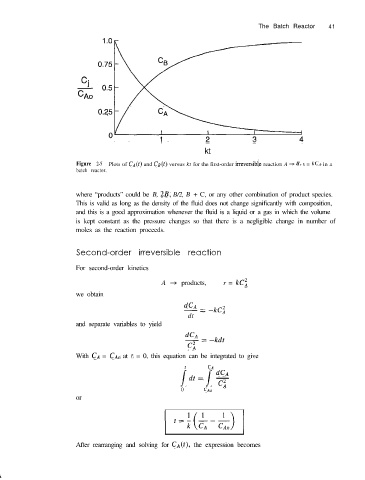
Figure 2-5 Plots of CA(~) and C,(t) versus kt for the first-order irreversible reaction A + B, I = kc,.+ in a
batch reactor.
where “products” could be B, 2B, B/2, B + C, or any other combination of product species.
This is valid as long as the density of the fluid does not change significantly with composition,
and this is a good approximation whenever the fluid is a liquid or a gas in which the volume
is kept constant as the pressure changes so that there is a negligible change in number of
moles as the reaction proceeds.
Second-order irreversible reaction
For second-order kinetics
A -+ products, r = kc:
we obtain
dC.+t
-= -kC;
dt
and separate variables to yield
With CA = CA,-, at t = 0, this equation can be integrated to give
f CA
dC.4
dt= -
s s G
0 CAo
or
After rearranging and solving for CA(t), the expression becomes