Page 59 - The engineering of chemical reactions
P. 59
The Batch Reactor 43
We now separate the equation to yield
C,” dCA = -kdt
This can be integrated
CA f
C,” dCA = - kdt
s s
CA” 0
to obtain _
-,; 1 (c,“+’ - ci:+‘) = -kt
WI
C,(t) = CA,[l + (n - l)kC’&‘tl”“‘“’
This equation is valid for any irreversible reaction of order n, but it cannot be used for n = 1
because the expression becomes indeterminate.
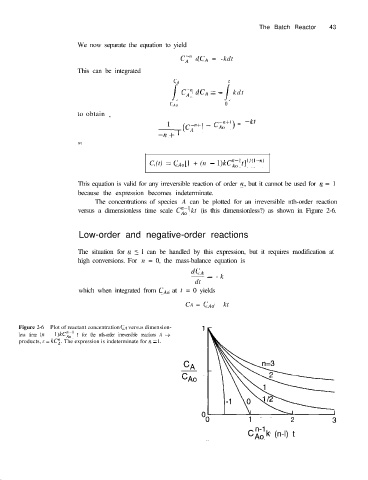
The concentrations of species A can be plotted for an irreversible nth-order reaction
versus a dimensionless time scale Ci,’ kt (is this dimensionless?) as shown in Figure 2-6.
Low-order and negative-order reactions
The situation for n 5 1 can be handled by this expression, but it requires modification at
high conversions. For n = 0, the mass-balance equation is
dC.+t
-= - k
dt
which when integrated from CA,, at t = 0 yields
CA = C.z,c, - kt
Figure 2-6 Plot of reactant concentration CA versus dimension-
less time (n - l)kCi;’ t for the nth-order irreversible reactions A --f
products, r = kC$. The expression is indeterminate for n = 1,
1 2
Clzk (n-l) t