Page 243 - The Mechatronics Handbook
P. 243
0066_frame_C12 Page 13 Wednesday, January 9, 2002 4:22 PM
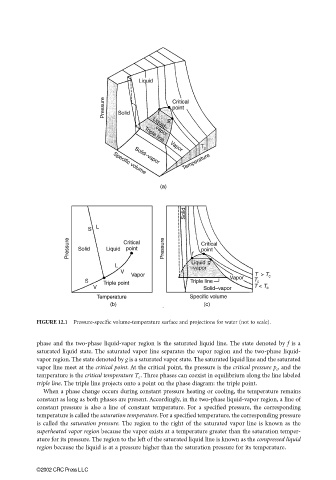
FIGURE 12.1 Pressure-specific volume-temperature surface and projections for water (not to scale).
phase and the two-phase liquid-vapor region is the saturated liquid line. The state denoted by f is a
saturated liquid state. The saturated vapor line separates the vapor region and the two-phase liquid-
vapor region. The state denoted by g is a saturated vapor state. The saturated liquid line and the saturated
vapor line meet at the critical point. At the critical point, the pressure is the critical pressure p c , and the
temperature is the critical temperature T c . Three phases can coexist in equilibrium along the line labeled
triple line. The triple line projects onto a point on the phase diagram: the triple point.
When a phase change occurs during constant pressure heating or cooling, the temperature remains
constant as long as both phases are present. Accordingly, in the two-phase liquid-vapor region, a line of
constant pressure is also a line of constant temperature. For a specified pressure, the corresponding
temperature is called the saturation temperature. For a specified temperature, the corresponding pressure
is called the saturation pressure. The region to the right of the saturated vapor line is known as the
superheated vapor region because the vapor exists at a temperature greater than the saturation temper-
ature for its pressure. The region to the left of the saturated liquid line is known as the compressed liquid
region because the liquid is at a pressure higher than the saturation pressure for its temperature.
©2002 CRC Press LLC