Page 262 - Thermodynamics of Biochemical Reactions
P. 262
262 Mathernatica Solutions to Problems
' S/mol
0.2 0.4 0.6 0.8 1
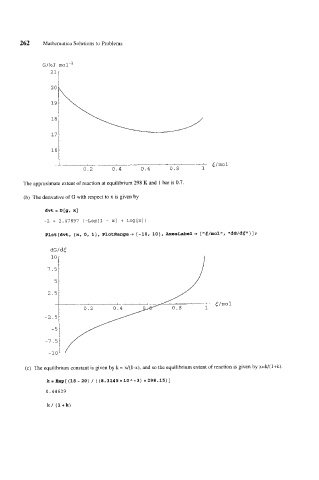
The approximate extent of reaction at equilibrium 298 K and 1 bar is 0.7.
(b) The derivative of G with respect to x is given by
dvt = D[g, X]
-2 + 2.47897 (-LOg[l - XI + Log [XI )
Plot [dvt, {x, 0, l), PlotRange -f {-lo, 101, AxesLabel+ {llg/molrl, "dG/dC"}] i
10:
7.5:
5:
2.5:
' Elmo1
1
-2.5:
-5:
-7.5:
-
-10
(c) The equilibrium constant is given by k = d(1-x), and so the equilibrium extent of reaction is given x=k/(l+k).
by
and
k
=
so
reaction
extent
of
the
equilibrium
given
constant
equilibrium
is
d(1-x),
-
=
/
]
k = Exp[ (18 - 20) / ((8.3145 *lo"-3) *298.15) ]
((8.3145
k
*10"-3)
20)
*298.15)
Exp[
(18
0.44629
0.44629