Page 267 - Thermodynamics of Biochemical Reactions
P. 267
Chemical Equilibrium in One Phase Systems 267
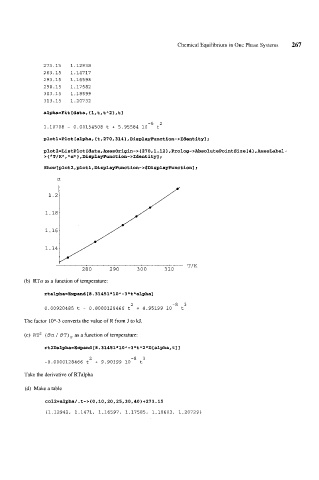
273.15 1.12938
283.15 1.14717
293.15 1.16598
298.15 1.17582
303.15 1.18599
313.15 1.20732
alpha=Fit[data,Cl,t,tA2>,tl
-6 2
1.10708 - 0.00154508 t + 5.95584 10 t
a
T/K
280 290 300 310
(b) RTa as a function of temperature:
rtalpha=Expand[8.31451*10A-3*t*alphal
2 -8 3
0.00920485 t - 0.0'000128466 t + 4.95199 10 t
The factor 10"-3 converts the value of R from J to kJ.
(c) RT~ 8 a / 8 T) as a function of temperature:
(
rt2Dalpha=Expand[8.31451*10A-3*tA2*D[alpha,tlJ
2 -8 3
-0.0000128466 t + 9.90399 10 t
Take the derivative of RTalpha
(d) Make a table
col2=alpha/.t->{0,10,20,25,30,40~+273.15
{1.12942, 1.1471, 1.16597, 1.17585, 1.18603, 1.20729)