Page 275 - Thermodynamics of Biochemical Reactions
P. 275
Chemical Equilibrium in One Phase Systems 275
Y
1
0.8
0.6
0.4
0.2
- I /M
0.05 0.1 0.15 0.2 0.25
At 40 "C
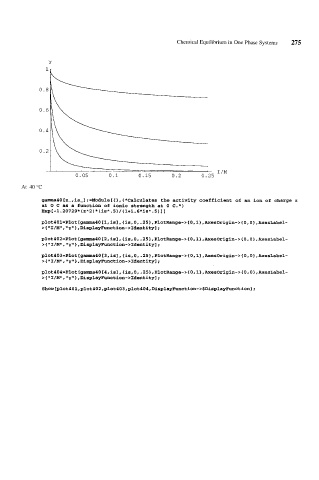
gamma40[z-,is-]:=Module[{},(*Calculates the activity coefficient of an ion of charge z
at 0 C as a function of ionic strength at 0 C.*)
Exp[-1.20729*(zA2)*(isA.5)/(l+l.6*isA.5)II
glot401=Plot[gamma40[1,is],{is,0,.25},PlotRange-~{O,1},AxesOrigin-~{O,O},AxesLabel-
> { "1 /MI1, "y" 1, Di splayhnct ion- >Identity] ;
glot402=Plot[gamma40[2,is],{is,0,.25},PlotRange-~{O,1~,AxesOrigin-~~O.O~.~e~Lab~~-
> { 1' I /MI', y" } , Di splayFunc t ion- >Ident i ty1;
1s
plot403=Plot[gamma40[3,~sl,{~s,0..25~,PlotRange->{O,l~,AxesOrigin->~O,O},AxesLabel-
> { I /MI1 , y 1 , Di SplayFunc t ion- >Identity 1;
glot404=Plot[ganrma4O[4,is],{is,0,.25},PlotRange-~~O,l},AxesOrigin-~~OrO~,AxesLabel-
>{ lmI/M1l, miyim} ,DisplayFunction->Identity1 ;