Page 566 - Bird R.B. Transport phenomena
P. 566
546 Chapter 18 Concentration Distributions in Solids and in Laminar Flow
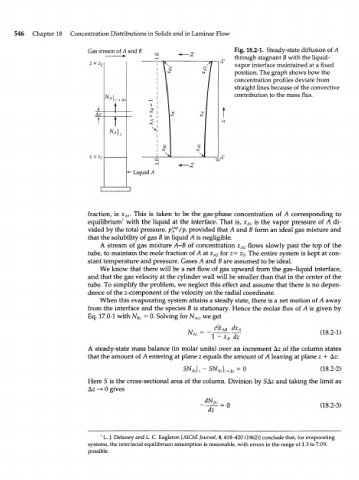
Gas stream of A and В Fig. 18.2-1. Steady-state diffusion of A
through stagnant В with the liquid-
vapor interface maintained at a fixed
position. The graph shows how the
concentration profiles deviate from
straight lines because of the convective
contribution to the mass flux.
fraction, is x . This is taken to be the gas-phase concentration of A corresponding to
M
equilibrium 1 with the liquid at the interface. That is, x Al is the vapor pressure of A di-
a?
vided by the total pressure, p\ /p, provided that A and В form an ideal gas mixture and
that the solubility of gas В in liquid A is negligible.
A stream of gas mixture A-B of concentration x A2 flows slowly past the top of the
tube, to maintain the mole fraction of A at x A2 for z= z . The entire system is kept at con-
2
stant temperature and pressure. Gases A and В are assumed to be ideal.
We know that there will be a net flow of gas upward from the gas-liquid interface,
and that the gas velocity at the cylinder wall will be smaller than that in the center of the
tube. To simplify the problem, we neglect this effect and assume that there is no depen-
dence of the z-component of the velocity on the radial coordinate.
When this evaporating system attains a steady state, there is a net motion of A away
from the interface and the species В is stationary. Hence the molar flux of A is given by
Eq. 17.0-1 with N Bz = 0. Solving for N , we get
Az
dx
A (18.2-1)
1 - х л dz
A steady-state mass balance (in molar units) over an increment Az of the column states
that the amount of A entering at plane z equals the amount of A leaving at plane z + Az:
SN L - SN A (18.2-2)
A
Here S is the cross-sectional area of the column. Division by SAz and taking the limit as
Az —» 0 gives
dN
Az = 0 (18.2-3)
dz
1 L. J. Delaney and L. C. Eagleton [AIChE Journal, 8, 418^120 (1962)] conclude that, for evaporating
systems, the interfacial equilibrium assumption is reasonable, with errors in the range of 1.3 to 7.0%
possible.