Page 586 - Bird R.B. Transport phenomena
P. 586
566 Chapter 18 Concentration Distributions in Solids and in Laminar Flow
If the catalytically active surface were all exposed to the stream of concentration c AR/
then the species A would not have to diffuse through the pores to a reaction site. The
molar rate of conversion would then be given by the product of the available surface and
the surface reaction rate:
W = {\irB?)(a){-Kc ) (18.7-12)
ARi0 AR
Taking the ratio of the last two equations, we get
j (18.7-13)
2
ф
in which ф = л/Ща7%^Я is the Thiele modulus^ encountered in §18.4. The quantity rj is
A
4
called the effectiveness factor?' It is the quantity by which W ARt0 has to be multiplied to ac-
count for the intraparticle diffusional resistance to the overall conversion process.
For nonspherical catalyst particles, the foregoing results may be applied approxi-
mately by reinterpreting R. We note that for a sphere of radius R the ratio of volume to
external surface is R/3. For nonspherical particles, we redefine JR in Eq. 18.7-13 as
R nonsph (18.7-14)
where V and S are the volume and external surface of a single catalyst particle. The ab-
P P
solute value of the conversion rate is then given approximately by
f
\W \ « V ak ;c ARVA (18.7-15)
AR
P
where
(18.7-16)
3A Z
in which the quantity Л = Л/к"а/ЯЬ (У /5 ) is a generalized modulus. '
2 3
А
Р
Р
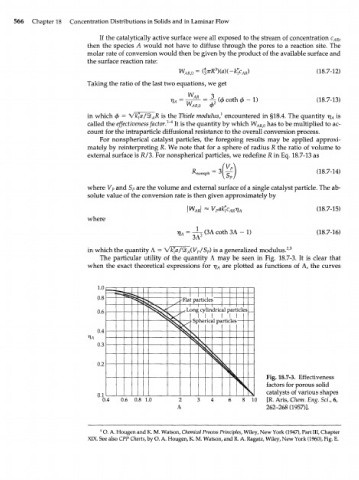
The particular utility of the quantity Л may be seen in Fig. 18.7-3. It is clear that
when the exact theoretical expressions for t] are plotted as functions of Л, the curves
A
1.0
1
0.8
J -Mat particles
0.6 cylindricalpartid BS
; Sp he ricedpa rticles
0.4
0.3 \
ч
0.2 4
4s Fig. 18.7-3. Effectiveness
factors
solid
for porous
0.1 ч catalysts of various shapes
0.4 0.6 0.8 1.0 2 8 10 [R. Aris, Chem. Eng. Sci., 6,
Л 262-268 (1957)].
4
O. A. Hougen and К. М. Watson, Chemical Process Principles, Wiley, New York (1947), Part III, Chapter
XIX. See also CPP Charts, by O. A. Hougen, K. M. Watson, and R. A. Ragatz, Wiley, New York (1960), Fig. E.