Page 581 - Bird R.B. Transport phenomena
P. 581
§18.5 Diffusion Into a Falling Liquid Film (Gas Absorption) 561
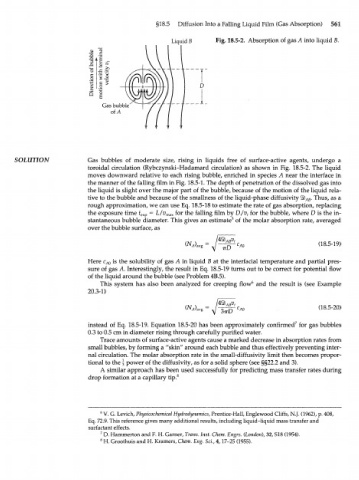
Liquid В Fig. 18.5-2. Absorption of gas A into liquid B.
о»
ion of t ~T
^p
i Gas bubble W / / ...1.
Dir D
o
f
Л
SOLUTION Gas bubbles of moderate size, rising in liquids free of surface-active agents, undergo a
toroidal circulation (Rybczynski-Hadamard circulation) as shown in Fig. 18.5-2. The liquid
moves downward relative to each rising bubble, enriched in species A near the interface in
the manner of the falling film in Fig. 18.5-1. The depth of penetration of the dissolved gas into
the liquid is slight over the major part of the bubble, because of the motion of the liquid rela-
tive to the bubble and because of the smallness of the liquid-phase diffusivity ЯЬ . Thus, as a
АВ
rough approximation, we can use Eq. 18.5-18 to estimate the rate of gas absorption, replacing
the exposure time t = L/v max for the falling film by D/v t for the bubble, where D is the in-
exp
stantaneous bubble diameter. This gives an estimate 5 of the molar absorption rate, averaged
over the bubble surface, as
(18.5-19)
Here c A0 is the solubility of gas A in liquid В at the interfacial temperature and partial pres-
sure of gas A. Interestingly, the result in Eq. 18.5-19 turns out to be correct for potential flow
of the liquid around the bubble (see Problem 4B.5).
This system has also been analyzed for creeping flow 6 and the result is (see Example
20.3-1)
(18.5-20)
instead of Eq. 18.5-19. Equation 18.5-20 has been approximately confirmed 7 for gas bubbles
0.3 to 0.5 cm in diameter rising through carefully purified water.
Trace amounts of surface-active agents cause a marked decrease in absorption rates from
small bubbles, by forming a "skin" around each bubble and thus effectively preventing inter-
nal circulation. The molar absorption rate in the small-diffusivity limit then becomes propor-
tional to the I power of the diffusivity, as for a solid sphere (see §§22.2 and 3).
A similar approach has been used successfully for predicting mass transfer rates during
drop formation at a capillary tip. 8
6 V. G. Levich, Physicochemical Hydrodynamics, Prentice-Hall, Englewood Cliffs, NJ. (1962), p. 408,
Eq. 72.9. This reference gives many additional results, including liquid-liquid mass transfer and
surfactant effects.
7 D. Hammerton and F. H. Garner, Trans. Inst. Chem. Engrs. {London), 32, S18 (1954).
8 H. Groothuis and H. Kramers, Chem. Eng. Sri., 4,17-25 (1955).