Page 577 - Bird R.B. Transport phenomena
P. 577
§18.4 Diffusion with a Homogeneous Chemical Reaction 557
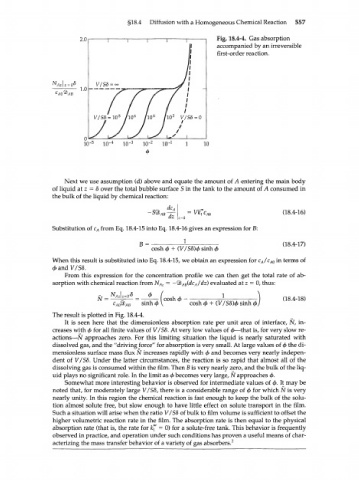
Fig. 18.4-4. Gas absorption
accompanied by an irreversible
first-order reaction.
1
V/S8 = 0
I
10
Next we use assumption (d) above and equate the amount of A entering the main body
of liquid at z = 8 over the total bubble surface S in the tank to the amount of A consumed in
the bulk of the liquid by chemical reaction:
(18.4-16)
2 = 6
Substitution of c from Eq. 18.4-15 into Eq. 18.4-16 gives an expression for B:
A
1
B = (18.4-17)
cosh ф + (У/Б8)ф sinh ф
When this result is substituted into Eq. 18.4-15, we obtain an expression for c /c in terms of
A A0
ф and V/SS.
From this expression for the concentration profile we can then get the total rate of ab-
sorption with chemical reaction from N = -%b (dc /dz) evaluated at z = 0, thus:
Az AB A
Ф 1
N = cosh ф (18.4-18)
с ЯЬ АВ sinh ф cosh ф + (У/Б8)ф sinh ф
А0
The result is plotted in Fig. 18.4-4.
It is seen here that the dimensionless absorption rate per unit area of interface, N, in-
creases with ф for all finite values of V/SS. At very low values of ф—that is, for very slow re-
actions—N approaches zero. For this limiting situation the liquid is nearly saturated with
dissolved gas, and the "driving force" for absorption is very small. At large values of ф the di-
mensionless surface mass flux N increases rapidly with ф and becomes very nearly indepen-
dent of V/S8. Under the latter circumstances, the reaction is so rapid that almost all of the
dissolving gas is consumed within the film. Then В is very nearly zero, and the bulk of the liq-
uid plays no significant role. In the limit as ф becomes very large, N approaches ф.
Somewhat more interesting behavior is observed for intermediate values of ф. It may be
noted that, for moderately large V/S8, there is a considerable range of ф for which N is very
nearly unity. In this region the chemical reaction is fast enough to keep the bulk of the solu-
tion almost solute free, but slow enough to have little effect on solute transport in the film.
Such a situation will arise when the ratio V/S8 of bulk to film volume is sufficient to offset the
higher volumetric reaction rate in the film. The absorption rate is then equal to the physical
absorption rate (that is, the rate for k'" = 0) for a solute-free tank. This behavior is frequently
observed in practice, and operation under such conditions has proven a useful means of char-
acterizing the mass transfer behavior of a variety of gas absorbers. 2