Page 574 - Bird R.B. Transport phenomena
P. 574
554 Chapter 18 Concentration Distributions in Solids and in Laminar Flow
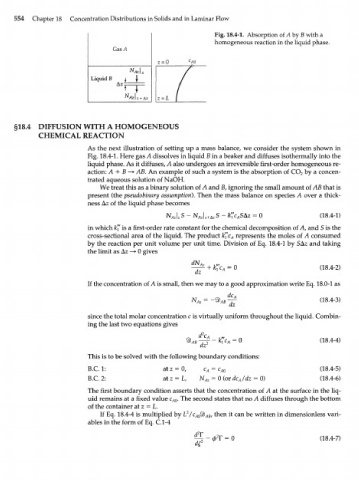
Fig. 18.4-1. Absorption of Л by В with a
homogeneous reaction in the liquid phase.
§18.4 DIFFUSION WITH A HOMOGENEOUS
CHEMICAL REACTION
As the next illustration of setting up a mass balance, we consider the system shown in
Fig. 18.4-1. Here gas A dissolves in liquid В in a beaker and diffuses isothermally into the
liquid phase. As it diffuses, A also undergoes an irreversible first-order homogeneous re-
action: A + В —> AB. An example of such a system is the absorption of CO by a concen-
2
trated aqueous solution of NaOH.
We treat this as a binary solution of A and B, ignoring the small amount of AB that is
present (the pseudobinary assumption). Then the mass balance on species A over a thick-
ness Az of the liquid phase becomes
N \ S - - k'"c SAz = 0 (18.4-1)
Az z A
in which k'" is a first-order rate constant for the chemical decomposition of A, and S is the
cross-sectional area of the liquid. The product k'"c represents the moles of A consumed
A
by the reaction per unit volume per unit time. Division of Eq. 18.4-1 by SAz and taking
the limit as Az —» 0 gives
+ k'l'c = 0 (18.4-2)
dz A
If the concentration of A is small, then we may to a good approximation write Eq. 18.0-1 as
dc
дг = -qt — A (18.4-3)
Л2 AB
since the total molar concentration с is virtually uniform throughout the liquid. Combin-
ing the last two equations gives
2
„ d c ,„,
A
(18.4-4)
This is to be solved with the following boundary conditions:
B.C.I: atz = 0, c = c (18.4-5)
AO
B.C. 2: at z = L, N A = 0 (or dcjdz = 0) (18.4-6)
Az
The first boundary condition asserts that the concentration of A at the surface in the liq-
uid remains at a fixed value c . The second states that no A diffuses through the bottom
A0
of the container at z = L.
2
If Eq. 18.4-4 is multiplied by L /C Q% , then it can be written in dimensionless vari-
A
AB
ables in the form of Eq. C.l-4
2
^ - ф Т = О (18.4-7)