Page 589 - Bird R.B. Transport phenomena
P. 589
Problems 569
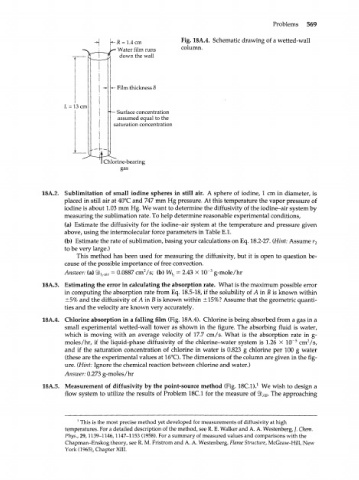
-R = 1.4 cm Fig. 18A.4. Schematic drawing of a wetted-wall
- Water film runs column.
down the wall
- Film thickness 8
L = 13 cm
— Surface concentration
assumed equal to the
saturation concentration
Chlorine-bearing
gas
18A.2o Sublimitation of small iodine spheres in still air. A sphere of iodine, 1 cm in diameter, is
placed in still air at 40°C and 747 mm Hg pressure. At this temperature the vapor pressure of
iodine is about 1.03 mm Hg. We want to determine the diffusivity of the iodine-air system by
measuring the sublimation rate. To help determine reasonable experimental conditions,
(a) Estimate the diffusivity for the iodine-air system at the temperature and pressure given
above, using the intermolecular force parameters in Table E.I.
(b) Estimate the rate of sublimation, basing your calculations on Eq. 18.2-27. (Hint: Assume r 2
to be very large.)
This method has been used for measuring the diffusivity, but it is open to question be-
cause of the possible importance of free convection.
2
Answer: (a) 2>,_ = 0.0887 cm /s; (b) W = 2.43 X 10" 3 g-mole/hr
h
2 air
18A.3. Estimating the error in calculating the absorption rate. What is the maximum possible error
in computing the absorption rate from Eq. 18.5-18, if the solubility of Л in В is known within
±5% and the diffusivity of Л in В is known within ±15%? Assume that the geometric quanti-
ties and the velocity are known very accurately.
18A.4. Chlorine absorption in a falling film (Fig. 18A.4). Chlorine is being absorbed from a gas in a
small experimental wetted-wall tower as shown in the figure. The absorbing fluid is water,
which is moving with an average velocity of 17.7 cm/s. What is the absorption rate in g-
2
5
moles/hr, if the liquid-phase diffusivity of the chlorine-water system is 1.26 X 10~ cm /s,
and if the saturation concentration of chlorine in water is 0.823 g chlorine per 100 g water
(these are the experimental values at 16°C). The dimensions of the column are given in the fig-
ure. (Hint: Ignore the chemical reaction between chlorine and water.)
Answer: 0.273 g-moles/hr
18A.5. Measurement of diffusivity by the point-source method (Fig. 18C.1). 1 We wish to design a
flow system to utilize the results of Problem 18C.1 for the measure of ЯЬ . The approaching
АВ
1
This is the most precise method yet developed for measurements of diffusivity at high
temperatures. For a detailed description of the method, see R. E. Walker and A. A. Westenberg, /. Chetn.
Phys., 29,1139-1146,1147-1153 (1958). For a summary of measured values and comparisons with the
Chapman-Enskog theory, see R. M. Fristrom and A. A. Westenberg, Flame Structure, McGraw-Hill, New
York (1965), Chapter XIII.