Page 593 - Bird R.B. Transport phenomena
P. 593
Problems 573
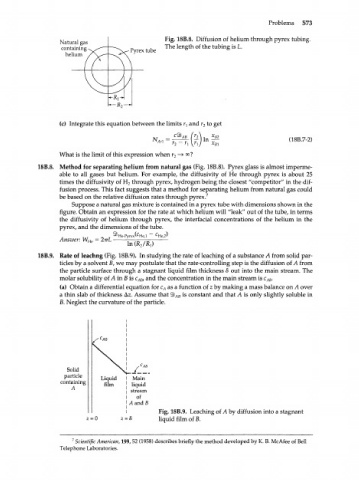
Natural gas Fig. 18B.8. Diffusion of helium through pyrex tubing.
containing Pyrex tube The length of the tubing is L.
helium
(c) Integrate this equation between the limits r and r to get
1
2
_ сЯЬ АВ (r 2 \ x B2
(18B.7-2)
What is the limit of this expression when r —» °°?
2
18B.8. Method for separating helium from natural gas (Fig. 18B.8). Pyrex glass is almost imperme-
able to all gases but helium. For example, the diffusivity of He through pyrex is about 25
times the diffusivity of H through pyrex, hydrogen being the closest "competitor" in the dif-
2
fusion process. This fact suggests that a method for separating helium from natural gas could
be based on the relative diffusion rates through pyrex. 7
Suppose a natural gas mixture is contained in a pyrex tube with dimensions shown in the
figure. Obtain an expression for the rate at which helium will "leak" out of the tube, in terms
the diffusivity of helium through pyrex, the interfacial concentrations of the helium in the
pyrex, and the dimensions of the tube.
^ )
Answer:
18B.9. Rate of leachng (Fig. 18B.9). In studying the rate of leaching of a substance A from solid par-
ticles by a solvent B, we may postulate that the rate-controlling step is the diffusion of A from
the particle surface through a stagnant liquid film thickness 8 out into the main stream. The
molar solubility of A in В is c , and the concentration in the main stream is c .
A0
A8
(a) Obtain a differential equation for c as a function of z by making a mass balance on A over
A
a thin slab of thickness Az. Assume that ЯЬ АВ is constant and that A is only slightly soluble in
B. Neglect the curvature of the particle.
Solid
particle Liquid Main
containing film liquid
A stream
of
Л and В
Fig. 18B.9. Leaching of A by diffusion into a stagnant
2 = 0 liquid film of B.
7 Scientific American, 199, 52 (1958) describes briefly the method developed by К. В. McAfee of Bell
Telephone Laboratories.