Page 716 - Bird R.B. Transport phenomena
P. 716
696 Chapter 22 Interphase Transport in Nonisothermal Mixtures
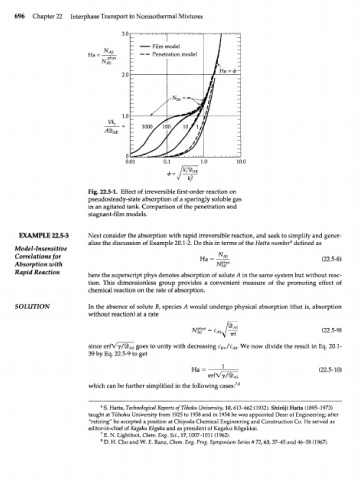
3.0
Film model
" A O
На = - - — — Penetration model
2.0
1.0
Vk
r
_ 1000
Л
АВ
0.01 10.0
Ф =
Fig. 22.5-1. Effect of irreversible first-order reaction on
pseudosteady-state absorption of a sparingly soluble gas
in an agitated tank. Comparison of the penetration and
stagnant-film models.
EXAMPLE 22.5-3 Next consider the absorption with rapid irreversible reaction, and seek to simplify and gener-
6
alize the discussion of Example 20.1-2. Do this in terms of the Hatta number defined as
Model-Insensitive
Correlations for Ha = (22.5-8)
Absorption with N5!? 5
Rapid Reaction here the superscript phys denotes absorption of solute A in the same system but without reac-
tion. This dimensionless group provides a convenient measure of the promoting effect of
chemical reaction on the rate of absorption.
SOLUTION In the absence of solute B, species A would undergo physical absorption (that is, absorption
without reaction) at a rate
= c A (22.5-9)
since eri\/y/4b AS goes to unity with decreasing c /c . We now divide the result in Eq. 20.1-
Bx
A0
39 by Eq. 22.5-9 to get
1
(22.5-10)
7 8
which can be further simplified in the following cases: : '
S. Hatta, Technological Reports ofTbhoku University, 10, 613-662 (1932). Shiroji Hatta (1895-1973)
6
taught at Tohoku University from 1925 to 1958 and in 1954 he was appointed Dean of Engineering; after
"retiring" he accepted a position at Chiyoda Chemical Engineering and Construction Co. He served as
editor-in-chief of Kagaku Kogaku and as president of Kagaku Kogakkai.
7
E. N. Lightfoot, Chem. Eng. Sci., 17,1007-1011 (1962).
D. H. Cho and W. E. Ranz, Chem. Eng. Prog. Symposium Series # 72, 63, 37^5 and 46-58 (1967).
8