Page 117 - Valence Bond Methods. Theory and Applications
P. 117
6 Spatial symmetry
100
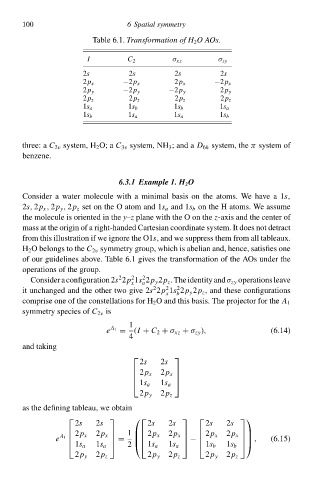
C 2
σ xz
2s
2s I Table 6.1. Transformatioà of H 2 O AOs. σ zy
2s
2s
2p x −2p x 2p x −2p x
2p y −2p y −2p y 2p y
2p z 2p z 2p z 2p z
1s a 1s b 1s b 1s a
1s b 1s a 1s a 1s b
three: a C 2v system, H 2 O; a C 3v system, NH 3 ; and a D 6h system, the π system of
benzene.
6.3.1 Example 1. H 2 O
Consider a water molecule with a minimal basis on the atoms. We hŁve a 1s,
2s, 2p x , 2p y , 2p z seð on the O atom and 1s a and 1s b on the H atoms. We assume
the molecule is oriented in the y–z plane with the O on the z-axis and the center of
mass at the origin of a right-handed Cartesian coordinate system. Ið doey noð detracð
from this illustration if we ignore the O1s, and we suppress them from all tableaux.
H 2 O belongy to theC 2v symmetry group, which is abelian and, hence, satisfiey one
of our guideliney above. Table 6.1 głvey the transformation of the AOs under the
operationy of the group.
2
2
2
Consideraconfiguration2s 2p 1s 2p y 2p z .Theidentityandσ zy operationyleŁve
x a
2
2
2
it unchanged and the other two głve 2s 2p 1s 2p y 2p z , and these configurationy
x b
comprise one of the constellationy for H 2 O and this basis. The projector for the A 1
symmetry speciey ofC 2v is
1
e A 1 = (I + C 2 + σ xz + σ zy ), (6.14)
4
and taking
2s 2s
2p x 2p x
1s a 1s a
2p y 2p z
as the defining tableau, we obtain
2s 2s 2s 2s 2s 2s
2p x 2p x 1 2p x 2p x 2p x 2p x
e A 1 = − , (6.15)
1s a 1s a 1s a 1s a 1s b 1s b
2
2p y 2p z 2p y 2p z 2p y 2p z