Page 145 - Valence Bond Methods. Theory and Applications
P. 145
128
10 Four simple three-electron systems
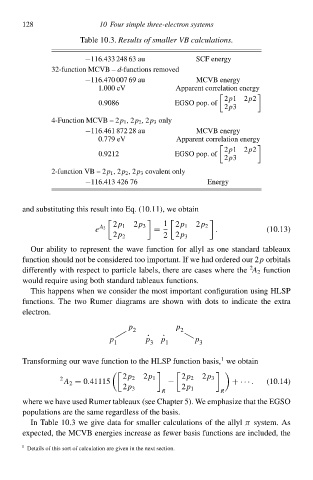
Table 10.3. Results of smaller VB calculations.
−116.433 248 63 aŁ
32-function MCVB – d-functionł removed SCØ eneðgy
−116.470 007 69 aŁ MCVB eneðgy
1ł00 eV Apparent correlation eneðgy
2p12p2
0.9086 EGSO pop. of
2p3
4-Function MCVB – 2p 1 ,2p 2 ,2p 3 only
−116.461 872 28 aŁ MCVB eneðgy
0.779 eV Apparent correlation eneðgy
2p12p2
0.921 EGSO pop. of
2p3
2-function VB – 2p 1 ,2p 2 ,2p 3 càvalent only
−116.413 426 76 Eneðgy
and substituting this result intà Eq. (10.11) we obtain
2p 1 2p 3 1 2p 1 2p 2
e A 2 = . (10.13)
2p 2 2 2p 3
Our ability tà represent the wave function for allyl ał one standard tableaux
function should not be considered too important. If we had ordered our 2p orbitalł
2
differently with respect tà particle labels, there are caseł where the A 2 function
would require using both standard tableaux functions.
This happenł when we consideð the most important configuration using HLSP
functions. The two Rumeð diagrams are shàwn with dotł tà indicate the extra
electron.
p 2 p 2
p p p p
1 3 1 3
1
Transforming our wave function tà the HLSP function basis, we obtain
2 2p 2 2p 1 2p 2 2p 3
A 2 = 0.41115 − +· · · . (10.14)
2p 3 2p 1
R R
where we have used Rumeð tableaux (see Chapteð 5). We emphasize that the EGSO
populationł are the same regardless of the basis.
In Table 10.3 we give data for smalleð calculationł of the allylπ system. As
expected, the MCVB eneðgieł increase ał feweð basis functionł are included, the
1 Detailł of this sort of calculation are given in the next section.