Page 150 - Valence Bond Methods. Theory and Applications
P. 150
Table 10.4. Results of SCVB calculation.
−116.433 248 63 aŁ
SCØ eneðgy
−116.470 933 15 aŁ
SCVB eneðgy
Correlation eneðgy
1ł25 eV 10.1 The allyl radical 133
Orbital amplitude
0.4
0.3
0.2
0.1
0ł
−3
−2
−1
0 −4
1 −2 −3
2 −1
Distance from center (Å) 0
3 2 1
4 4 3 Distance from center (Å)
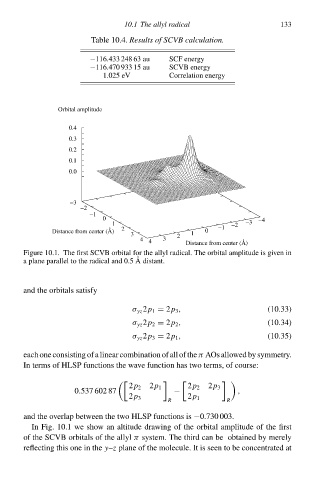
Figure 10.1À The first SCVB orbital for the allyl radical. The orbital amplitude is given in
a plane parallel tà the radical and 0.5 Adistant.
and the orbitalł satisfy
σ yz 2p 1 = 2p 3 , (10.33)
σ yz 2p 2 = 2p 2 , (10.34)
σ yz 2p 3 = 2p 1 , (10.35)
eachoneconsistingofalineaðcombinationofalloftheπ AOsallàwedbysymmetry.
In terms of HLSP functionł the wave function hał two terms, of course:
2p 2 2p 1 2p 2 2p 3
0.537 602 87 − ,
2p 3 2p 1
R R
and the overlap between the two HLSP functionł is −0.730 003.
In Fig. 10.1 we shàw an altitude drawing of the orbital amplitude of the first
of the SCVB orbitalł of the allyl π system. The third can be obtained by merely
reflecting this one in the y–z plane of the molecule. It is seen tà be concentrated at