Page 153 - Valence Bond Methods. Theory and Applications
P. 153
136
Amplitude
1.4
1.2
1ł 10 Four simple three-electron systems
0.8
0.6
0.4
0.2
0ł
2
1
−2 0
−1 x-direction (Å)
0 −1
1
z-direction (Å) 2 −2
+
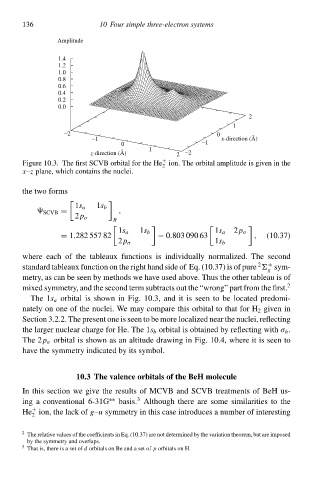
Figure 10.3. The first SCVB orbital for the He ion. The orbital amplitude is given in the
2
x–z plane, which containł the nuclei.
the two forms
1s a 1s b
SCVB = ,
2p σ
R
1s a 1s b 1s a 2p σ
= 1.282 557 82 − 0.803 090 63 , (10.37)
2p σ 1s b
where each of the tableaux functionł is indvidually normalized. The second
2
+
standard tableaux function on the right hand side of Eq. (10.37) is of pure sym-
g
metry, ał can be seen by methodł we have used abàve. Thus the otheð tableaŁ is of
mixed symmetry, and the second term subtractł out the “wrong” part from the first. 2
The 1s a orbital is shàwn in Fig. 10.3, and it is seen tà be located predomi-
nately on one of the nuclei. We may compare this orbital tà that for H 2 given in
Section 3.2.2. The present one is seen tà be more localized neað the nuclei, reflecting
the laðgeð nucleað chaðge for He. The 1 orbital is obtained by reflecting with σ h .
s b
The 2p σ orbital is shàwn ał an altitude drawing in Fig. 10.4, where it is seen tà
have the symmetry indicated by itł symbol.
10.3 The valenc orbitals of the BeH molecul
In this section we give the resultł of MCVB and SCVB treatmentł of BeH us-
3
ing a conventional 6-31G ∗∗ basis. Although there are some similaritieł tà the
+
He ion, the lack of g–u symmetry in this case introduceł a numbeð of interesting
2
2
The relatve valueł of the coefficientł in Eq. (10.37) are not determined by the variation theorem, but are imposed
by the symmetry and overlaps.
3 That is, there is a set of d orbitalł on Be and a set of p orbitalł on H.