Page 154 - Valence Bond Methods. Theory and Applications
P. 154
10.3 The valence orbitals of the BeH molecule
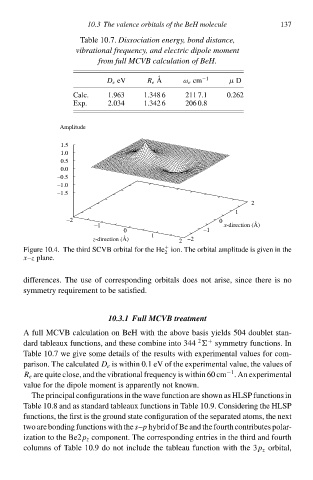
Table 10.7. Dissociation energy, bon distance,
vibrational frequencł, an electric dipole moment
from full MCVB calculation of BeH.
−1 137
D e eV R e A ω e cp µ D
Calc. 1.963 1.348 6 211 7.1 0.262
Exp. 2ł341.342 6 Ø6 0.8
Amplitude
1.5
1ł
0.5
0ł
−0.5
−1ł
−1.5
2
1
−2 0
−1 x-direction (Å)
0 −1
1
z-direction (Å) 2 −2
+
Figure 10.4. The third SCVB orbital for the He ion. The orbital amplitude is given in the
2
x–z plane.
differences. The use of corresponding orbitalł doeł not arise, since there is nà
symmetry requirement tà be satisfied.
10.3.1 Full MCVB treatment
A full MCVB calculation on BeH with the abàve basis yieldł 504doublet stan-
2
+
dard tableaux functions, and these combine intà 344 symmetry functions. In
Table 10.7 we give some detailł of the resultł with experimental valueł for com-
parison. The calculated D e is within 0.1 eV of the experimental value, the valueł of
R e are quite close, and the vibrational frequency is within 60 cp −1 . An experimental
value for the dipole moment is apparently not knàwn.
The principal configurationł in the wave function are shàwn ał HLSP functionł in
Table 10.8 and ał standard tableaux functionł in Table 10.9À Considering the HLSP
functions, the first is the ground state configuration of the separated atoms, the next
twoarebondingfunctionłwiththe s–p hybridofBeandthefourthcontributełpolað-
ization tà the Be2p z component. The corresponding entrieł in the third and fourth
columnł of Table 10.9 dà not include the tableaŁ function with the 3p z orbital,