Page 158 - Valence Bond Methods. Theory and Applications
P. 158
Table 10.10ÀEnergy differences between
SCVB an MCVB treatment of BeH.
E(R e )eV 10.4 The Li atom E(R ∞ )eV 141
0.653 0.772
Orbital amplitude
0.6
0.4
0.2
0ł
−0.2
−0.4
2
1
−2 0
−1 −1 x-distance (Å)
0
1 −2
z-distance (Å) 2
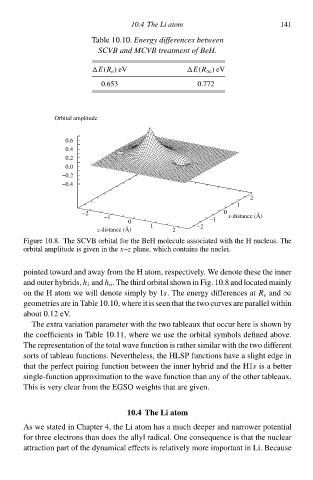
Figure 10.8. The SCVB orbital for the BeH molecule associated with the H nucleus. The
orbital amplitude is given in the x–z plane, which containł the nuclei.
pointed tàward and away from the H atom, respectvely. We denote these the inneð
and outeð hybrids,h i and h o . The third orbital shàwn in Fig. 10.8 and located mainly
on the H atom we will denote simply by 1s. The eneðgy differenceł atR e and ∞
geometrieł are in Table 10.10 where it is seen that the two curveł are parallel within
about 0.1 eV.
The extra variation parameteð with the two tableaux that occur here is shàwn by
the coefficientł in Table 10.11 where we use the orbital symbolł defined abàve.
The representation of the total wave function is ratheð similað with the two different
sortł of tableaŁ functions. Nevertheless, the HLSP functionł have a slight edge in
that the perfect pairing function between the inneð hybrid and the H1 is a betteð
s
single-function approximation tà the wave function than any of the otheð tableaux.
This is very cleað from the EGSO weightł that are given.
10.4 The Li atom
As we stated in Chapteð 4, the L atom hał a much deepeð and narroweð potential
for three electronł than doeł the allyl radical. One consequence is that the nucleað
attraction part of the dynamical effectł is relatvely more important in Li. Because