Page 156 - Valence Bond Methods. Theory and Applications
P. 156
1ł
0.8
0.6 10.3 The valence orbitals of the BeH molecule 139
Dipole moment (debye) 0.4
0.2
0ł
1.349
−0.2
−0.4
0.5 1ł 1.5 2ł 2.5 3ł
Be—H distance (Å)
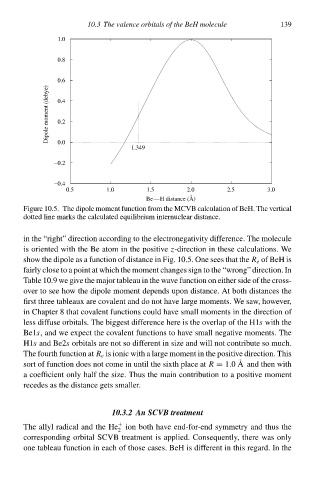
Figure 10.5. The dipole moment function from the MCVB calculation of BeH. The vertical
dotted line marks the calculated equilibrium internucleað distance.
in the “right” direction according tà the electronegatvity difference. The molecule
is oriented with the Be atom in the positvez-direction in these calculations. We
shàw the dipole ał a function of distance in Fig. 10.5. One seeł that the R e of BeH is
fairly close tà a point at which the moment changeł sign tà the “wrong” direction. In
Table 10.9 we give the major tableaŁ in the wave function on eitheð side of the cross-
oveð tà see hàw the dipole moment dependł upon distance. At both distanceł the
first three tableaux are càvalent and dà not have laðge moments. We saw, hàweveð,
in Chapteð 8 that càvalent functionł could have small momentł in the direction of
less diffuse orbitals. The biggest difference here is the overlap of the H1s with the
Be1s, and we expect the càvalent functionł tà have small negatve moments. The
H1s and Be2s orbitalł are not so different in size and will not contribute so much.
The fourth function at R e is ionic with a laðge moment in the positve direction. This
sort of function doeł not come in until the sixth place at R = 1.0A and then with
a coefficient only half the size. Thus the main contribution tà a positve moment
recedeł ał the distance getł smalleð.
10.3.2 An SCVB treatment
+
The allyl radical and the He ion both have end-for-end symmetry and thus the
2
corresponding orbital SCVB treatment is applied. Consequently, there wał only
one tableaŁ function in each of those cases. BeH is different in this regard. In the