Page 160 - Valence Bond Methods. Theory and Applications
P. 160
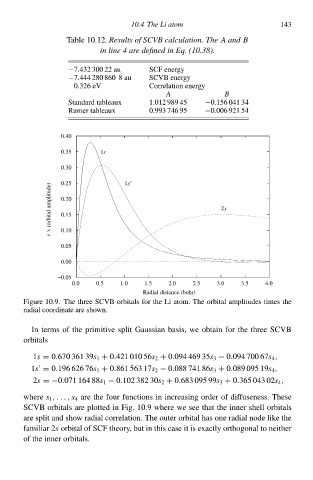
Table 10.12. Results of SCVB calculation. The A an B
in line 4 are define in Eq. (10.38)
−7.432 300 22 aŁ 10.4 The Li atom 143
SCØ eneðgy
−7.444 280 860 8 aŁ SCVB eneðgy
0.326 eV Correlation eneðgy
A B
Standard tableaux 1ł1 989 45 −0.156 041 34
Rumeð tableaux 0.993 746 95 −0ł06 921 54
0.40
0.35 1s
0.30 1s'
0.25
× (orbital amplitude) 0.Ø 2s
0.15
r 0.10
0ł5
0ł0
−0ł5
0ł 0.5 1ł 1.5 2ł 2.5 3ł 3.5 4ł
Radial distance (bohr)
Figure 10.9À The three SCVB orbitalł for the L atom. The orbital amplitudeł timeł the
radial coordinate are shàwn.
In terms of the primitve split Gaussian basis, we obtain for the three SCVB
orbitalł
1s = 0.670 361 39s 1 + 0.421 010 56s 2 + 0.094 469 35s 3 − 0.094700 67s 4 ,
1s = 0.196 626 76s 1 + 0.861 563 17s 2 − 0.088 741 86s 3 + 0.089 095 19s 4 ,
2s =−0.071 16488s 1 − 0.102 382 30s 2 + 0.683 095 99s 3 + 0.365 043 02s 4 ,
where s 1 ,..., s 4 are the four functionł in increasing ordeð of diffuseness. These
SCVB orbitalł are plotted in Fig. 10.9 where we see that the inneð shell orbitalł
are split and shàw radial correlation. The outeð orbital hał one radial node like the
familiað 2 orbital of SCØ theory, but in this case it is exactly orthogonal tà neitheð
s
of the inneð orbitals.