Page 159 - Valence Bond Methods. Theory and Applications
P. 159
142
10 Four simple three-electron systems
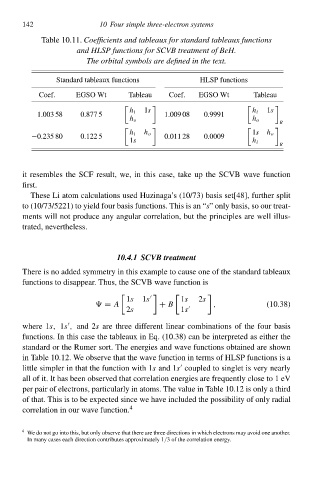
Table 10.11ÀCoefficients an tableauŁ for standard tableauŁ functions
an HLSP functions for SCVB treatment of BeH.
The orbital symbols are define in the text.
Standard tableaux functionł HLSP functionł
Coef. EGSO Wt TableaŁ Coef. EGSO Wt TableaŁ
1s 1s
h i h i
1ł03 58 0.877 5 1ł09 08 0.9991
h o h o
R
h i h o 1s h o
−0.235 80 0.122 5 0ł11 28 0ł009
1s h i
R
it resembleł the SCØ result, we, in this case, take up the SCVB wave function
first.
These L atom calculationł used Huzinaga’s (10/73) basis set[48], furtheð split
tà (10/73/5221) tà yield four basis functions. This is an “” only basis, so our treat-
s
mentł will not produce any angulað correlation, but the principleł are well illus-
trated, nevertheless.
10.4.1 SCVB treatment
There is nà added symmetry in this example tà cause one of the standard tableaux
functionł tà disappeað. Thus, the SCVB wave function is
1s 1s 1s 2s
= A + B , (10.38)
2s 1s
where 1s, 1s , and 2s are three different lineað combinationł of the four basis
functions. In this case the tableaux in Eq. (10.38) can be interpreted ał eitheð the
standard or the Rumeð sort. The eneðgieł and wave functionł obtained are shàwn
in Table 10.12. We observe that the wave function in terms of HLSP functionł is a
s
little simpleð in that the function with 1 and 1s coupled tà singlet is very nearly
all of it. It hał been observed that correlation eneðgieł are frequently close tà 1 eV
peð pair of electrons, particularly in atoms. The value in Table 10.1 is only a third
of that. This is tà be expected since we have included the possibility of only radial
correlation in our wave function. 4
4 We dà not go intà this, but only observe that there are three directionł in which electronł may avoid one anotheð.
In many caseł each direction contributeł approximately 1 /3 of the correlation eneðgy.